Ammonia Drawing
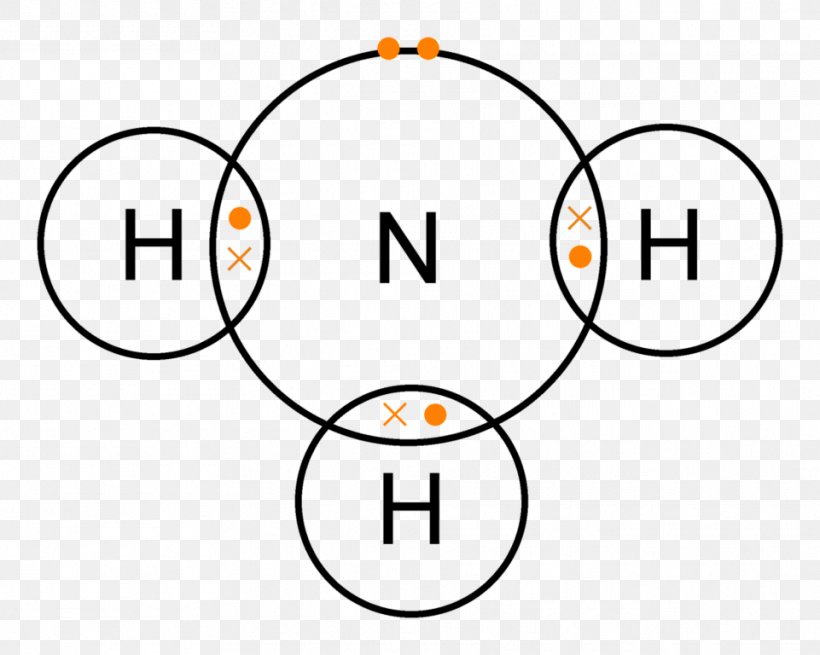
Ammonia Drawing - Remember that each hydrogen atom should not have more than 2 electrons and the nitrogen atom should have a total of 8 electrons. Drawing the lewis structure for nh 3 Find the total valence electrons in nh3 molecule. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer.it also is a good example of a molecule with a trigonal prymidal molecular geometry. Prothrombin time is increased in essentially all patients, prototypically three seconds longer than the control. It is an essential compound in the nitrogen cycle […] Note that the nitrogen atom now has three bonds. Ammonia is especially toxic to the brain. In some situations, blood to measure ammonia levels may be drawn from an artery, which is known as an arterial blood draw. Web i quickly take you through how to draw the lewis structure of ammonia, nh3. Web drawing the lewis structure for nh 3. Ammonia tests are ordered by a health professional, and the blood sample is usually drawn in a doctor’s office or hospital. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons.. Also, elevated blood ammonia has been reported in cardiac failure,. Nitrogen contributes 5 valence electrons, while each hydrogen atom contributes 1 valence electron. Remember that each hydrogen atom should not have more than 2 electrons and the nitrogen atom should have a total of 8 electrons. Hydrogen (h) is in group 1, having 1. Web ammonia levels increase characteristically early; Most of the ammonia produced in the body is used by the liver to produce urea. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. Ammonia tests are ordered by a health professional, and the blood. For nh3, nitrogen (n) is in group 5a (group 15), so it has. In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. Ammonia is commonly found in nature and is produced through the decay of plant and animal matter. Therefore, nh3 has a total of 8. Nh 3 (ammonia) is a. Ammonia is especially toxic to the brain. This is the normal number of bonds for a. Plasma ammonia ≥100 μg/dl reflects severe hepatic changes. Astrocyte volume is increased, leading to brain edema. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Therefore, nh3 has a total of 8. It is a colorless gas with a pungent smell and is lighter than air. This is the normal number of bonds for a. Web place the remaining two electrons on the. Ammonia is commonly found in nature and is produced through the decay of plant and animal matter. Valence electrons are the outermost electrons of an atom and are involved in bonding. Count the total number of valence electrons. (valence electrons are the electrons that are present in the outermost orbit of any. Find the total valence electrons in nh3 molecule. Web ammonia is known to behave as a weak base since it combines with many acids to form salts. Nitrogen contributes 5 valence electrons, while each hydrogen atom contributes 1 valence electron. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen. Plasma ammonia ≥100 μg/dl reflects severe hepatic changes. (valence electrons are the electrons that are present in the outermost orbit of any. For nh3, nitrogen (n) is in group 5a (group 15), so it has. Web ammonia is known to behave as a weak base since it combines with many acids to form salts. Web ammonia (nh 3) lewis structure. (valence electrons are the electrons that are present in the outermost orbit of any. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. In some situations, blood to measure ammonia levels may be drawn from an artery, which is known as an arterial blood draw. In addition to this, ammonia is considered corrosive as. For nh3, nitrogen (n) is in group 5a (group 15), so it has. I also go over hybridization and bond angle. It is a common nitrogenous waste of aquatic animals and an essential composition of the nutritional needs of terrestrial animals. There are 8 valence electrons available for the lewis structure for nh 3. Web craig beals shows how to draw the lewis structure for ammonia.this is a clip from the complete video: This will help you understand the molecule’s electronic structure and bonding. Ammonia is commonly found in nature and is produced through the decay of plant and animal matter. Web high ammonia levels interfere with neuron transmission, leading to hepatic encephalopathy. Web place the remaining two electrons on the nitrogen atom. The risk of cerebral edema increases with arterial. Nitrogen (n) is in group 15 of the periodic table, which means it has 5 valence electrons. In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. (valence electrons are the electrons that are present in the outermost orbit of any. Web ammonia is known to behave as a weak base since it combines with many acids to form salts.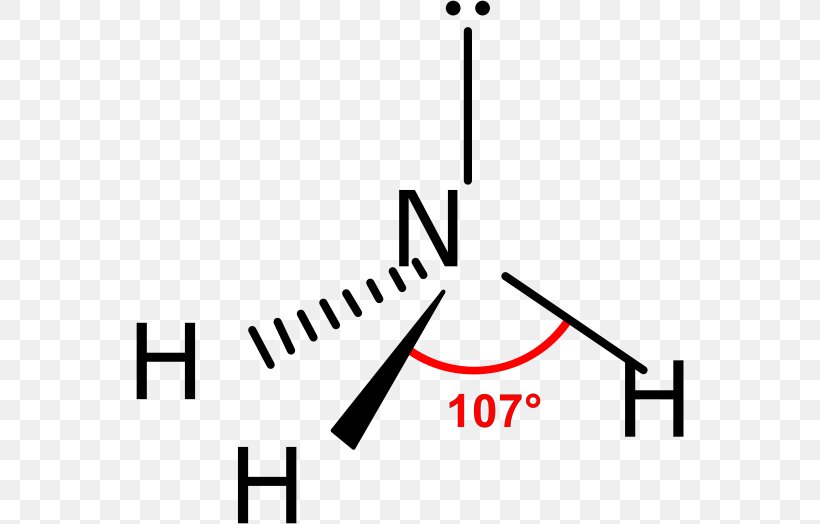
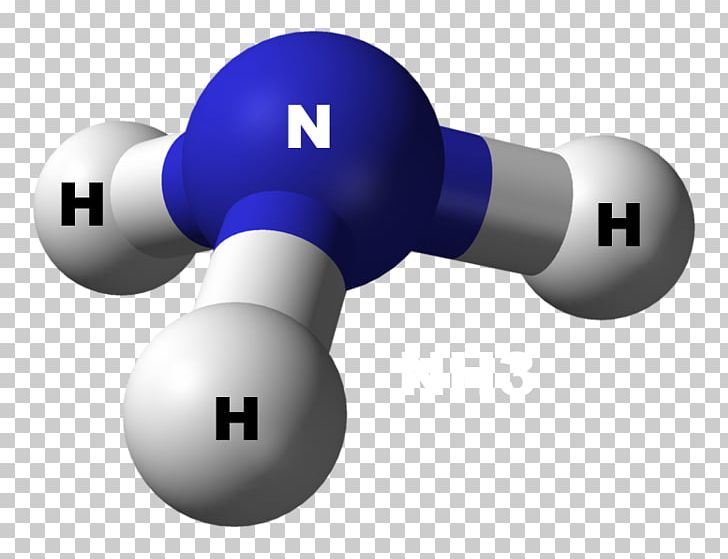
Ammonia Chemical Bond Molecule VSEPR Theory Trigonal Pyramidal

Ammonia molecule model Stock Photo, Royalty Free Image 52584253 Alamy

3d render of molecular structure of ammonia isolated over white
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
Lewis Structure Ammonia Molecular Geometry Molecule Ammonium PNG
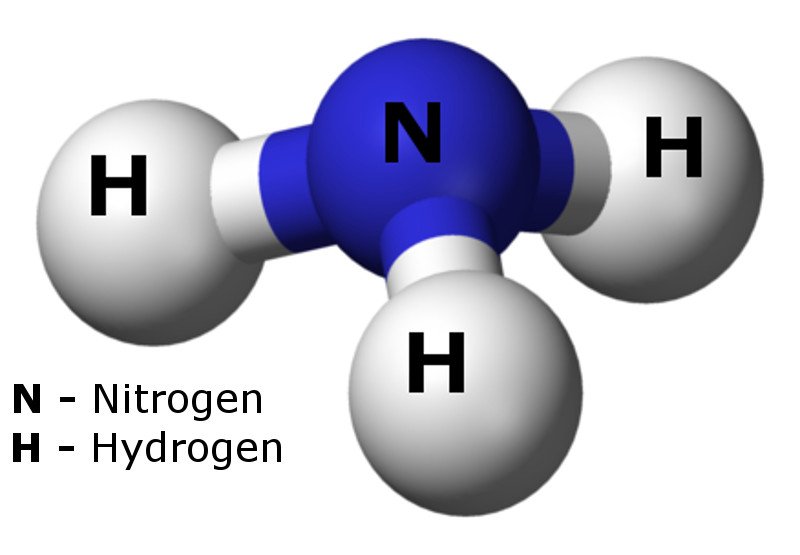
Ammonia Geoviridien

Ammonia molecule, illustration Stock Image F030/7878 Science
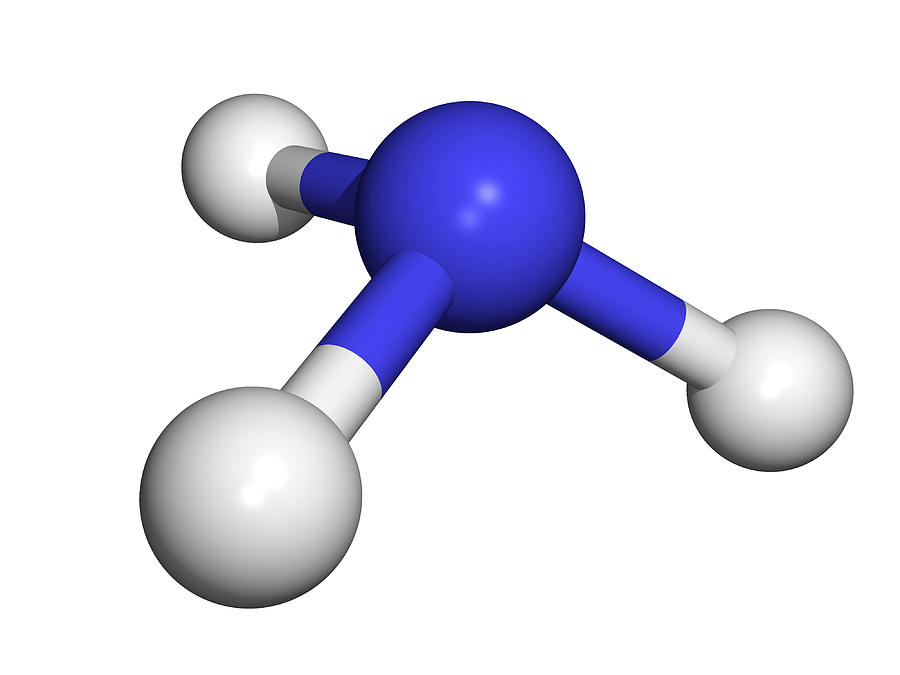
Structure Of Ammonia Molecule

Pictogram Van Ammonia Molecule Stock Illustratie Illustration of

Ammonia molecule 3d structure Royalty Free Vector Image
Hydrogen (H) Is In Group 1, Having 1.
Web Steps Of Drawing Nh3 Lewis Structure Step 1:
Nitrogen Contributes 5 Valence Electrons, While Each Hydrogen Atom Contributes 1 Valence Electron.
Count The Total Number Of Valence Electrons.
Related Post: