Draw Bondline Structures For C5H12
Draw Bondline Structures For C5H12 - C5h12 draw the lewis structure: Include wedges and dashes if they apply. Same skeleton (including h) same skeleton (excluding h) Thumb_up 100% transcribed image text: This problem has been solved! This molecule has all c’s bonded to one other c in a row. This problem has been solved! So, the molecular formula is c5h12. 1) there is a carbon at each junction (corner) and periphery. The condensed structural formula will be ch 2(ch 2)3ch 3. Notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. This molecule has all c’s bonded to one other c in a row. #1 draw a rough skeleton structure. This problem has been solved! Draw the skeletal formula of this molecule. So, the molecular formula is c5h12. Web we recommend you use a larger device to draw your structure. Notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Same skeleton (including h) same skeleton (excluding h) Draw the structure and give the systematic name for a compound with molecular formula c5h12 that has. We have c five h 12 c5 edge in the second part. #1 draw a rough skeleton structure. Draw the lewis structure for c5h12. Draw the lewis structure for c5h12. 1 answer junaid mirza apr 10, 2018 three are possible. 1 answer junaid mirza apr 10, 2018 three are possible. Thumb_up 100% transcribed image text: You'll get a detailed solution from a subject matter expert. And how many total hydrogens do we have? In chemistry, isomers are molecules with identical molecular formulas — that is, same number of atoms of each element — but distinct arrangements of atoms. The condensed structural formula will be ch 2(ch 2)3ch 3. And we'll start with the molecule we talked about in the bond line structure video, so. Draw the lewis structure for c5h12. 13k views 2 years ago. This problem has been solved! Web what are the structural isomers of c5h12o? The condensed formula for propanal is ch 3 ch 2 cho. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The structural formula will be ch 3ch 2ch 2ch 2ch 3. The condensed structural formula will be ch 2(ch 2)3ch 3. Draw the lewis structure for c5h12. Web what are the structural isomers of c5h12o? Web structural isomers in c5h12? How do i determine the molecular shape of a molecule? Web draw the bond line structures for c5h12. And how many total hydrogens do we have? Now let's draw all of the structural isomers that have the molecular formula c3h8o. So, let's look at this next bond line structure here, and let's focus in on our carbon. #1 draw a rough skeleton structure. Same skeleton (including h) same skeleton (excluding h) And we'll start with the molecule we talked about in the bond line structure video, so. The first is the seafood h 10 seafood and the second will be them. This problem has been solved! The structural formula will be ch 3ch 2ch 2ch 2ch 3. Web so we have a total of three structural isomers that have the molecular. These isomers have different chemical structures and molecular geometries, which give them distinct properties and behaviors. C5h12 draw the lewis structure: So, the molecular formula is c5h12. Web we recommend you use a larger device to draw your structure. You'll get a detailed solution from a subject matter expert. Include wedges and dashes if they apply. Trending now this is a popular solution! Thumb_up 100% transcribed image text: Draw the skeletal formula of this molecule. And none of the atoms has a lone pair. This problem has been solved! How is vsepr used to. There are 8 alcohol and 6 ether isomers. Draw the structure and give the systematic name for a compound with molecular formula c5h12 that has (c) one tertiary hydrogen. The left carbon and right carbon are attached with three hydrogen atoms, and the three center carbons are attached with two hydrogen atoms. Draw the condensed formula of this molecule. The condensed formula for propanal is ch 3 ch 2 cho. The structural formula will be ch 3ch 2ch 2ch 2ch 3. Build the model using the model set. You'll get a detailed solution from a subject matter expert. Include all lone pair electrons, if any exist.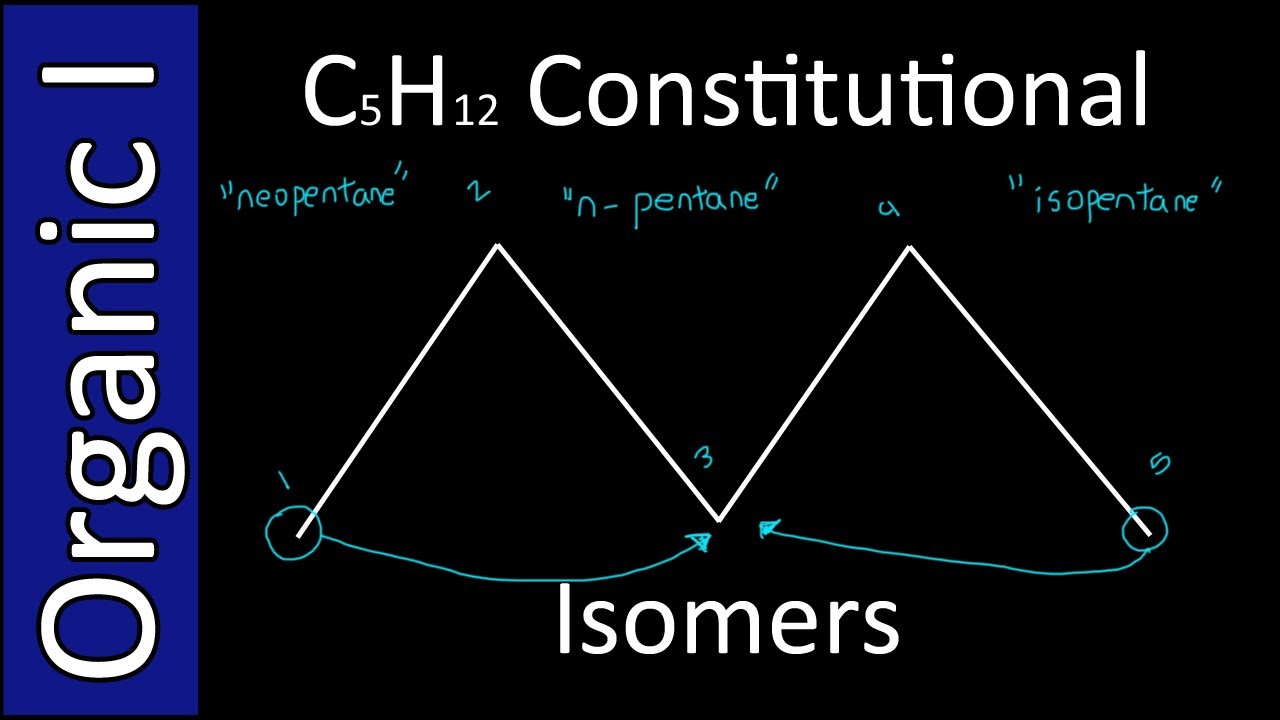
Draw The Structures Of All The Constitutional Isomers Of C5h12 Label
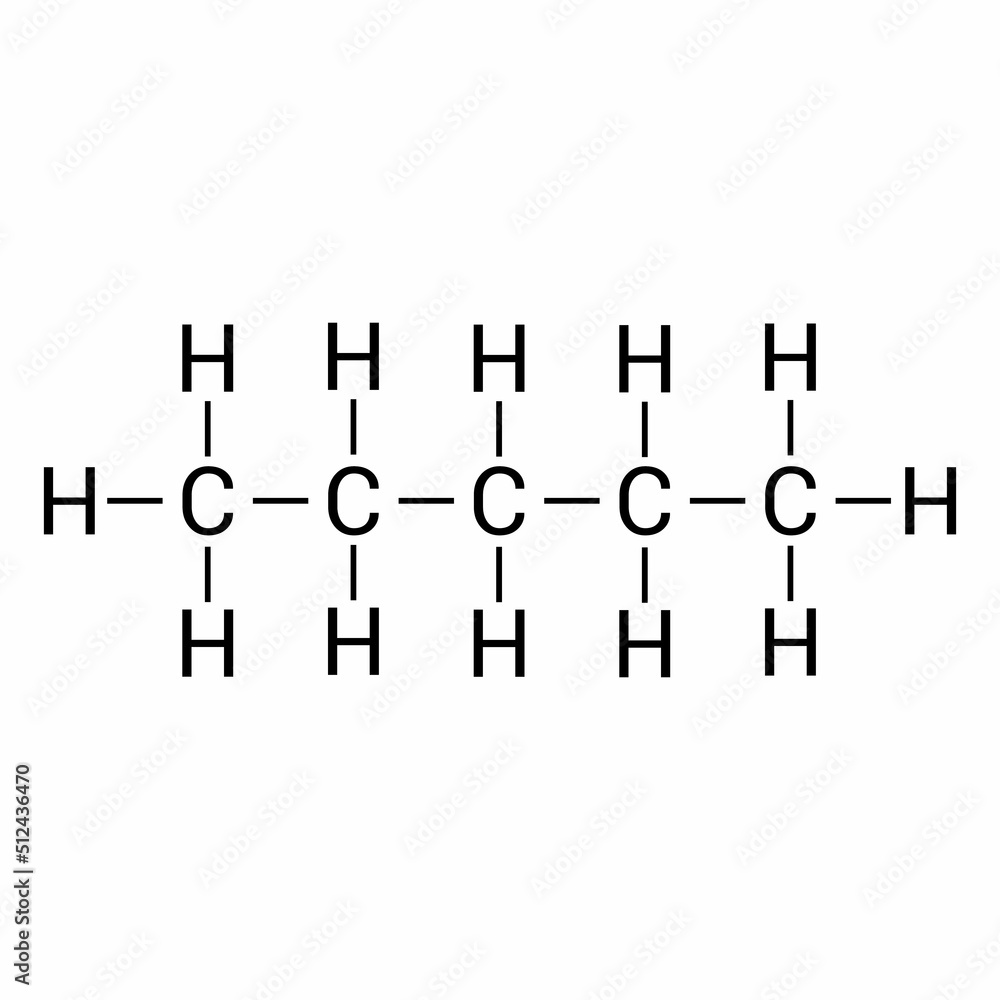
chemical structure of Pentane (C5H12) Stock Vector Adobe Stock

C5h12 Lewis Structure
SOLVEDDraw bondline structures for all constitutional isomers of C5 H12.

How to Draw the Lewis Dot Structure for C5H12 Pentane YouTube

C5h12 Lewis Structure
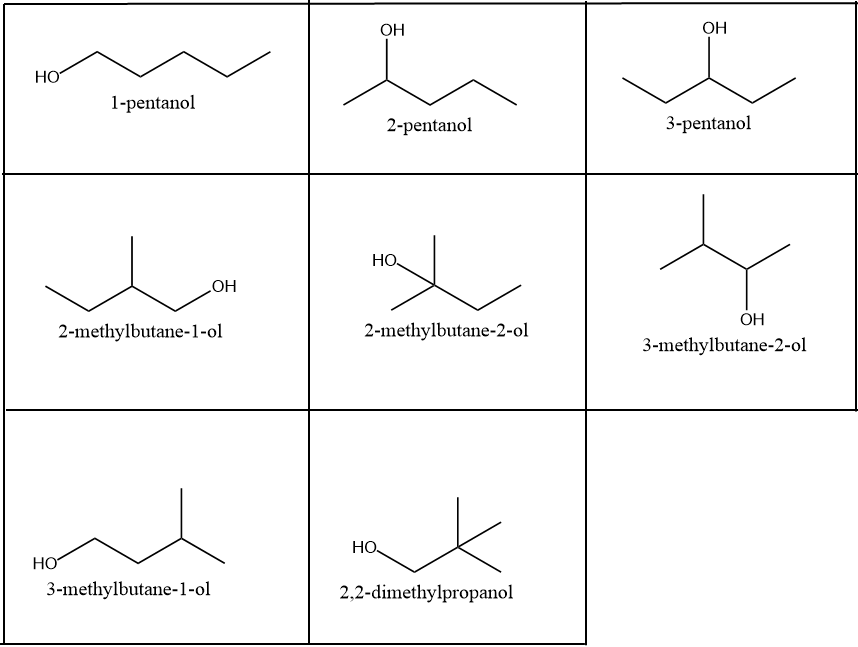
Draw The Structures And Write The Names Of All Possible Isomers Of A
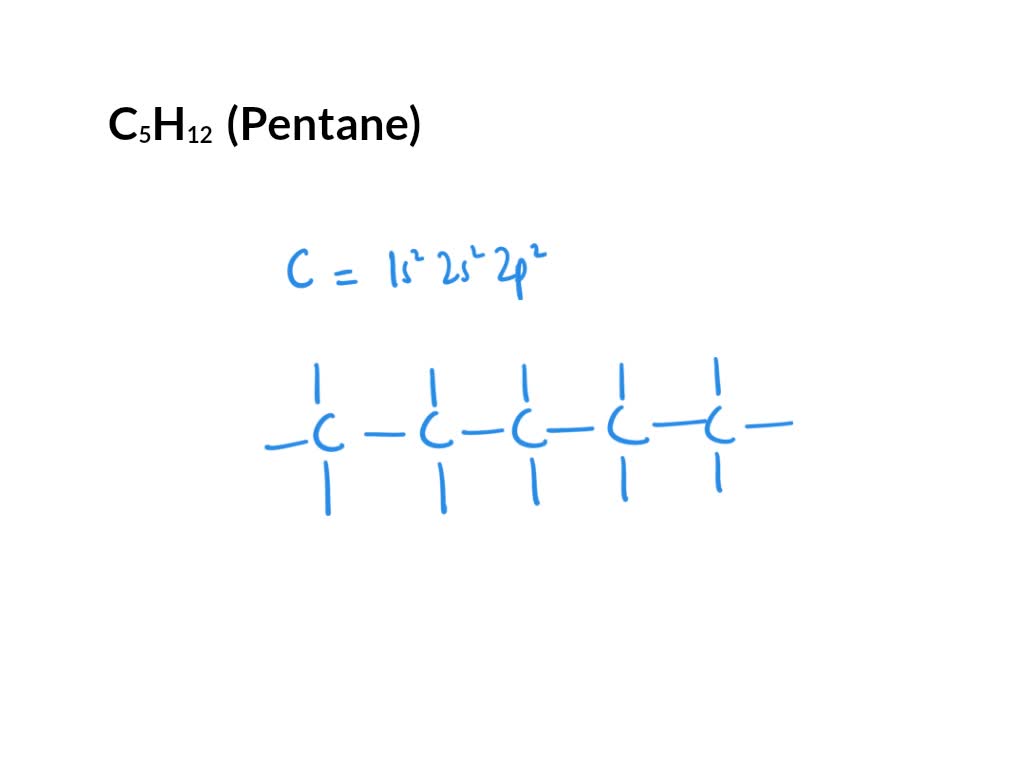
SOLVED Draw the Lewis structure for C5H12. Draw the skeletal formula

Lewis Structure Of C5h12
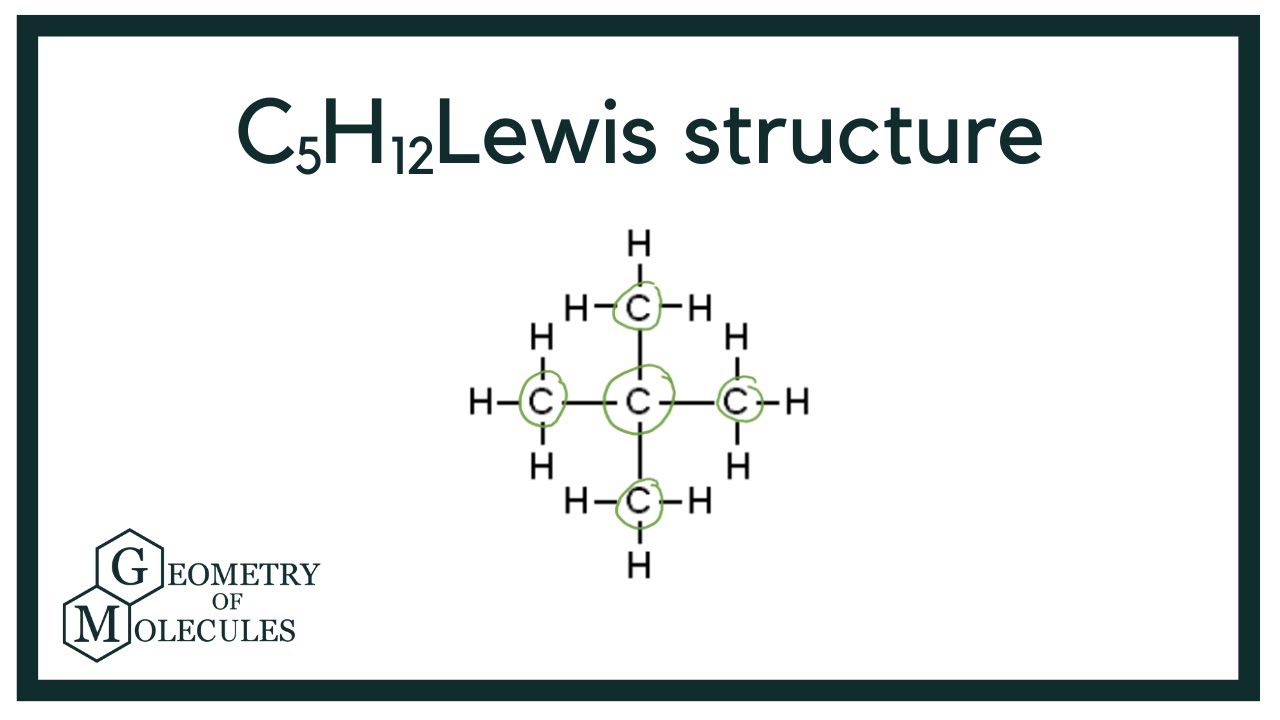
C5H12 Lewis Structure How to Draw the Lewis Structure for C5H12
Try Rotating The Device So That It Is In A Landscape Position.
So, The Molecular Formula Is C5H12.
846 Views 1 Year Ago Lewis Structure.
The Condensed Structural Formula Will Be Ch 2(Ch 2)3Ch 3.
Related Post: