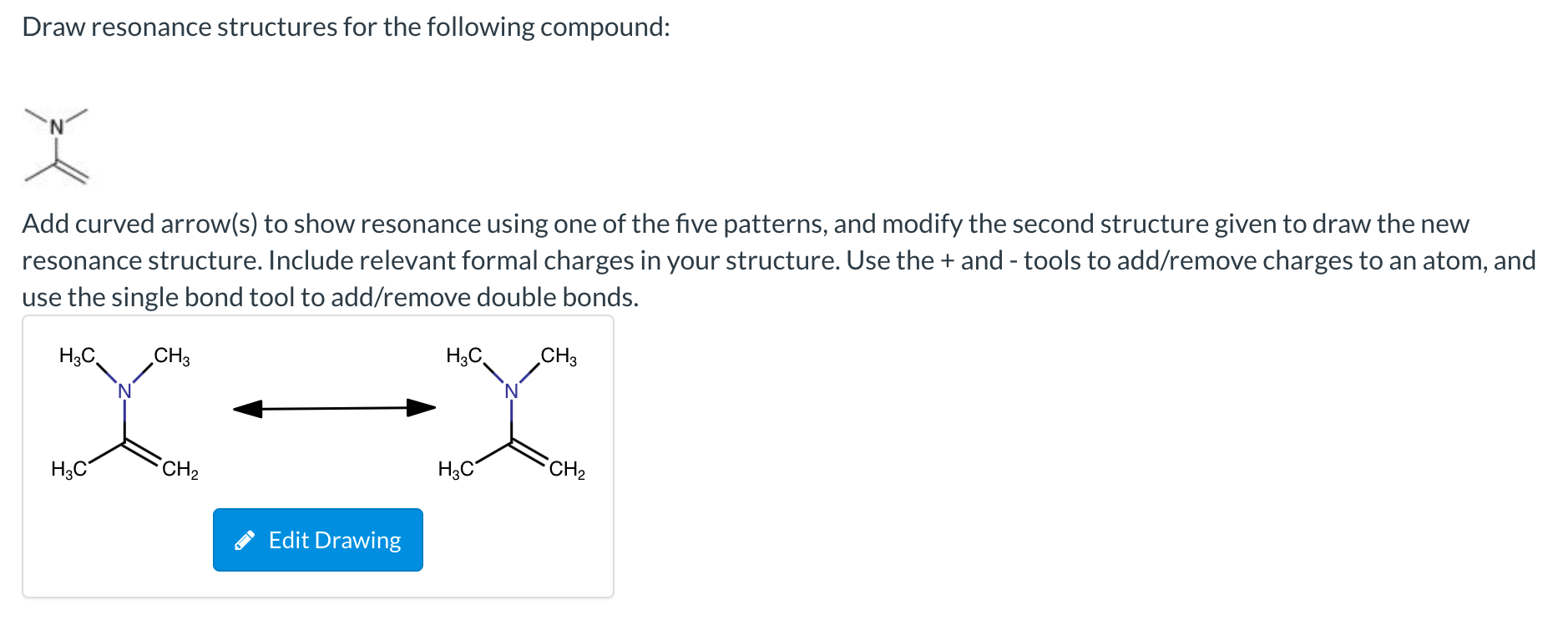
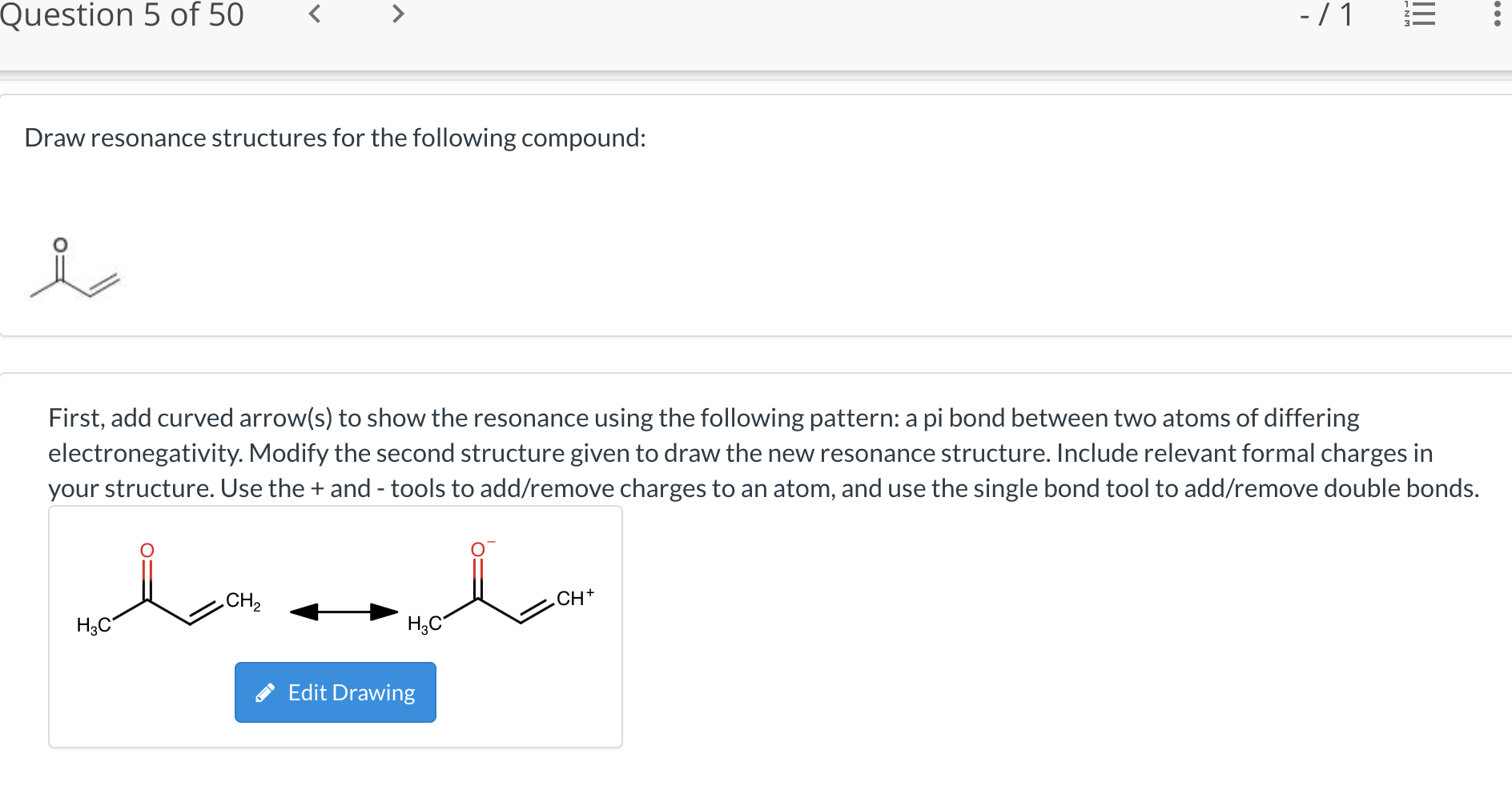
Draw Resonance Structures For The Following Compound
Draw Resonance Structures For The Following Compound - Web draw all possible resonance structures for the following free radical: Resonance is a mental exercise and method. (i) c h 2 = c h − c l. Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Step 1 first, add curved arrows) to show the resonance using the following pattern: The following rules should be helpful: Not all will obey the octet rule. The resonating structures of nitro benzene are. Ozone (o3) ozone has two major resonance structures that contribute equally to its overall hybrid structure. Web it is a superposition, in which a single molecule can behave like all three structures at the same time. (iii) c h 2 = c h − c | h = o. Not all will obey the octet rule. In resonance structures, it does not require to show. The following rules should be helpful: Web draw resonance structures for the following compound: There is only one π bond in this example, and no any lone pairs, so only the π electrons can be. In resonance structures, it does not require to show. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Resonance is a mental exercise and method. (i) c. Web draw all possible resonance structures for the following free radical: Draw sets of resonance structures for the following compounds. To draw all resonance structures, take the lewis structure we drawn by using vespr rule. Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Use curved arrows to depict the conversion. Resonance is a mental exercise and method. A pi bond between two atoms. Web draw all possible resonance structures for the following free radical: To draw all resonance structures, take the lewis structure we drawn by using vespr rule. The resonating structures of phenol are represented as: Web it is a superposition, in which a single molecule can behave like all three structures at the same time. Web it explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with all of the lone pairs, bonds, and partial charges. The resonating structures of phenol are represented as:. Not all will obey the octet rule. (b) the structure of c 6 h 5 no 2 is: Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Calculate the total number of valence electrons from each atom. When switching from general to organic chemistry, showing. Draw another resonance structure based on the given one. (iii) c h 2 = c h − c | h = o. Web (a) the structure of c 6 h 5 oh is: The resonating structures of nitro benzene are. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web how to draw resonance structures. Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. To draw all resonance structures, take the lewis structure we drawn by using vespr rule. In resonance structures,. (iii) c h 2 = c h − c | h = o. Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Draw sets of resonance structures for the following compounds. Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. In resonance. Web draw the resonance structures of the following compounds; (b) the structure of c 6 h 5 no 2 is: (iii) c h 2 = c h − c | h = o. Web draw all possible resonance structures for the following free radical: A pi bond between two atoms. Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. In resonance structures, it does not require to show. The resonating structures of phenol are represented as: Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Web it explains how to draw the resonance structures using curved arrow notation and how to draw the resonance hybrid as well with all of the lone pairs, bonds, and partial charges. A pi bond between two atoms. To draw all resonance structures, take the lewis structure we drawn by using vespr rule. When switching from general to organic chemistry, showing. Web draw the resonance structures of the following compounds; (ii) c h 2 = c h − c h = c h 2. Use curved arrows to depict the conversion. In many cases, a single. Determine all the pushable electron pairs and the places where the electrons. (b) the structure of c 6 h 5 no 2 is: Web (a) the structure of c 6 h 5 oh is: Web draw resonance structures for the following compound: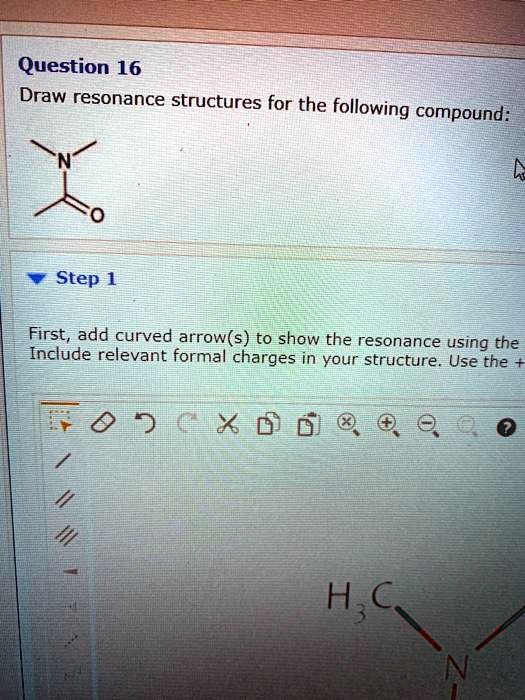
SOLVED Question 16 Draw resonance structures for the following
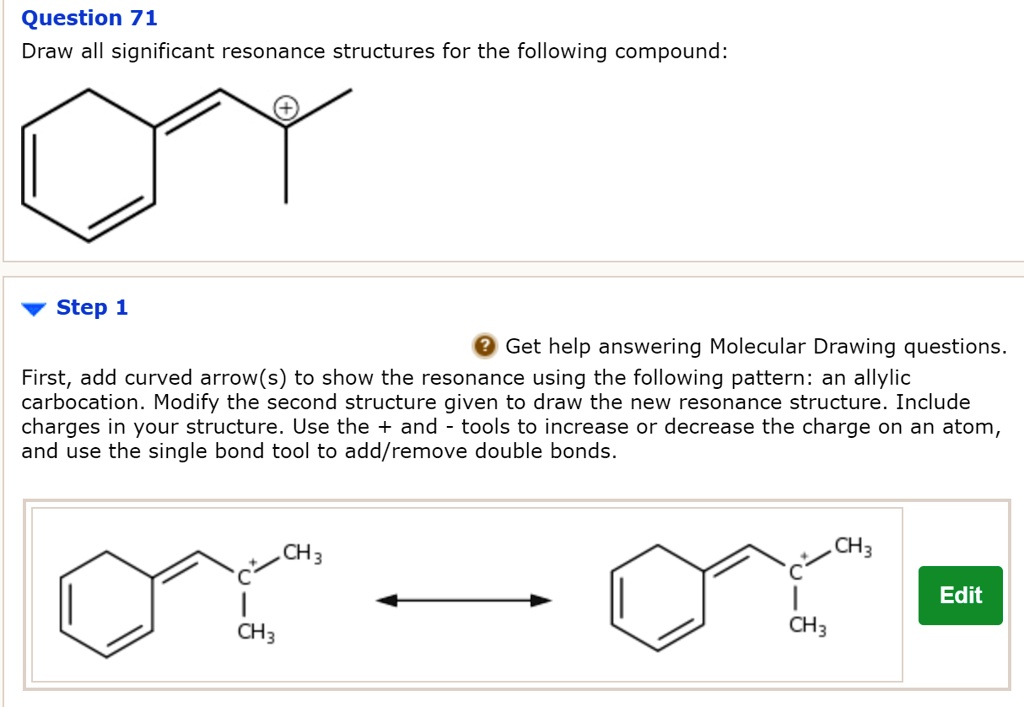
draw significant resonance structures for the following compound

1.3 Resonance Structures Organic Chemistry I
Solved Draw resonance structures for the following compound

Draw a Resonance Structure for the Compound Below.
[Solved] . Draw resonance structures for the following compounds. Add
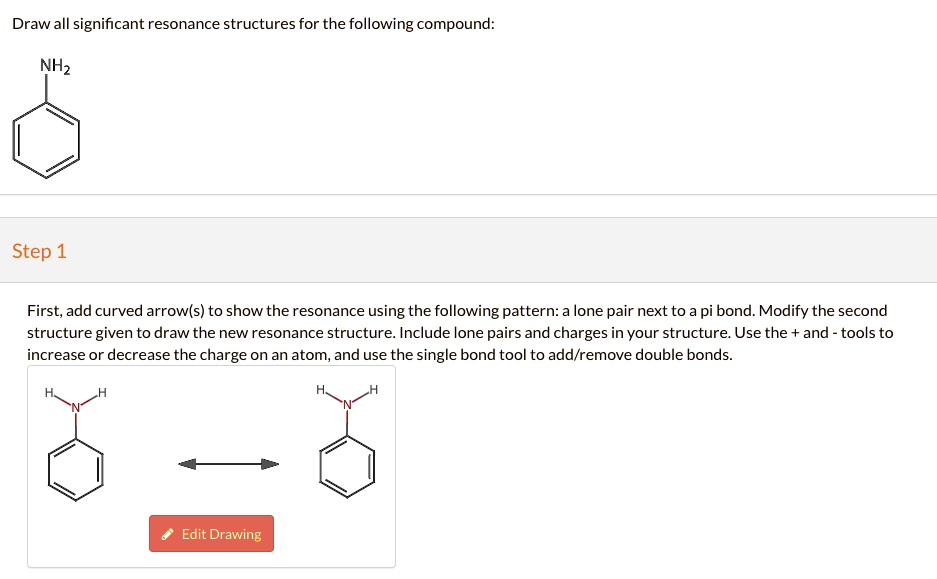
SOLVED Draw all significant resonance structures for the following
Solved Draw resonance structures for the following compound
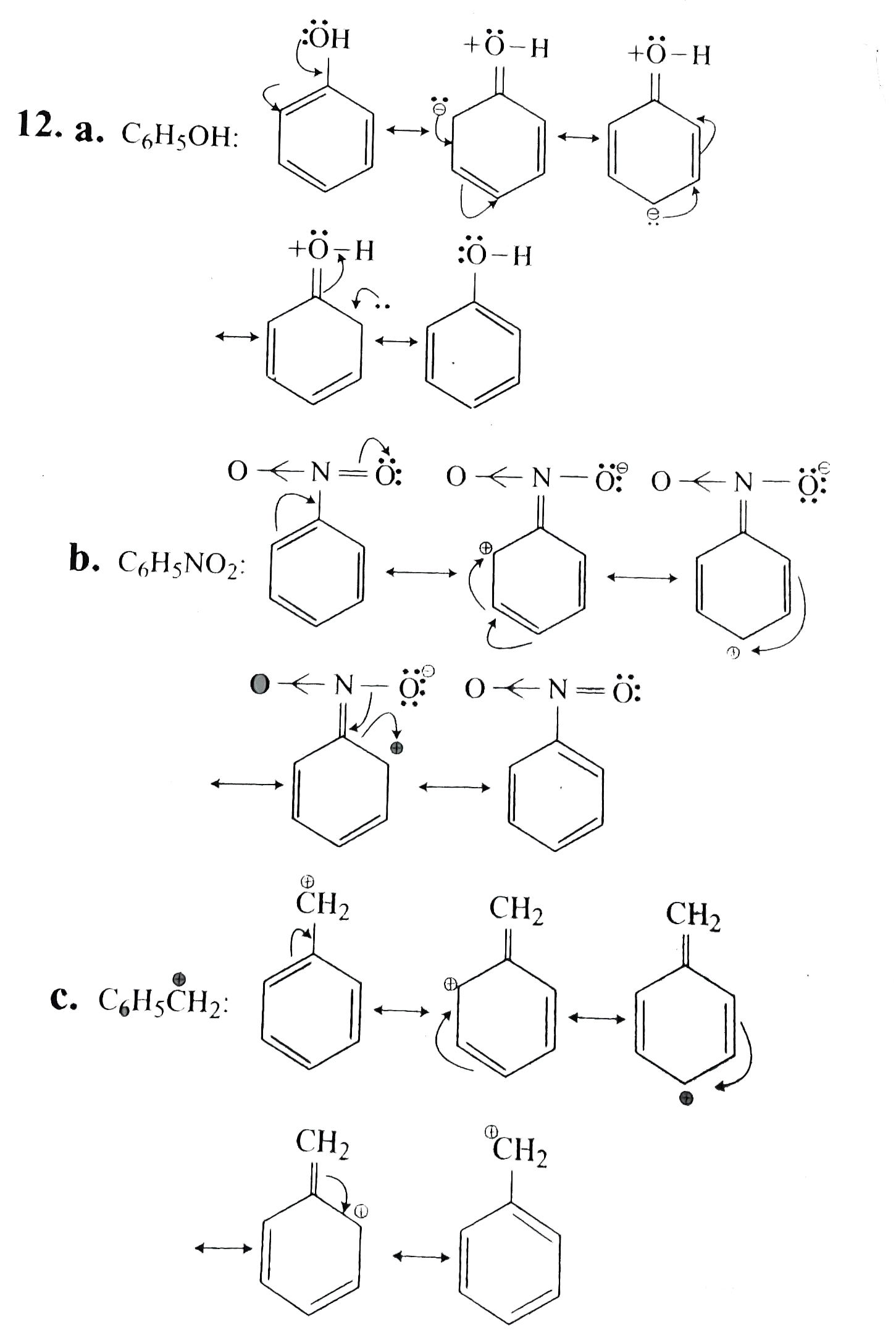
Draw the resonance structures for the following compounds. Show the el
![[Solved] Draw resonance structures for the follow](https://media.cheggcdn.com/study/abf/abfbc7e3-78c5-42b6-bd2d-d0b05735ba03/image.jpg)
[Solved] Draw resonance structures for the follow
Draw Another Resonance Structure Based On The Given One.
Calculate The Total Number Of Valence Electrons From Each Atom.
Resonance Is A Mental Exercise And Method.
There Is Only One Π Bond In This Example, And No Any Lone Pairs, So Only The Π Electrons Can Be.
Related Post: