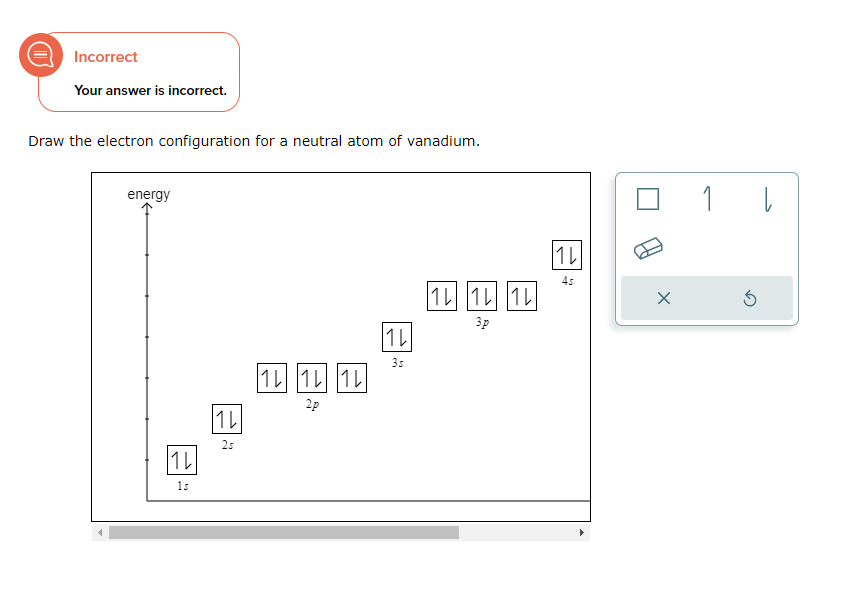
Draw The Electron Configuration For A Neutral Atom Of Vanadium
Draw The Electron Configuration For A Neutral Atom Of Vanadium - 1s2 2s2 2p6 3s2 3p6 3d3 4s2. Web full electron configuration of vanadium: Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide. [ar]4s 2 3d 3, 5. Web the electron configuration of vanadium is [ar]3d34s2. Note, although monatomic anions are isoelectronic to a nobel gas,. Web there are a set of general rules that are used to figure out the electron configuration of an atomic species: By knowing the electron configuration of an element, we can predict and. Titanium ← vanadium → chromium. Read ratings & reviewsshop our huge selectionfast shippingshop best sellers Web full electron configuration of vanadium: Read ratings & reviewsshop our huge selectionfast shippingshop best sellers List the subshells following the model shown in figure 1.6.3: Aufbau principle, hund's rule and the pauli. Web the electron configuration for a neutral atom of vanadium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d³. Web full electron configuration of vanadium: Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. First, write out the electron configuration for each parent atom. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide. Web here's the. Look at the periodic table and find an atomic number of oxygen, which is 8. Cr and cu, as well as cu and ag, are exceptions in the typical. Web here's the electron configuration for vanadium: Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide.. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Vanadium is defined as a chemical element belonging to the periodic table, it is part of group 5, it is represented by. Web you simply have additional electrons to the neutral atom, and add them the same way you would for an atom.. Electrons always fill in the lowest energy configuration possible. Look at the periodic table and find an atomic number of oxygen, which is 8. 1s² (2 electrons) 2s² (2 electrons) 2p⁶ (6 electrons) 3s² (2 electrons) 3p⁶ (6 electrons) 4s² (2 electrons) 3d³. By knowing the electron configuration of an element, we can predict and. Alternatively, this can be abbreviated. List the subshells following the model shown in figure 1.6.3: Alternatively, this can be abbreviated as [ar]4s²3d³, using the. 1s² (2 electrons) 2s² (2 electrons) 2p⁶ (6 electrons) 3s² (2 electrons) 3p⁶ (6 electrons) 4s² (2 electrons) 3d³. Web the electron configuration of vanadium is [ar]3d34s2. Web the electron configuration of an element is the arrangement of its electrons in. The electron configuration for a neutral atom of. List the subshells following the model shown in figure 1.6.3: Web there are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau principle, hund's rule and the pauli. Web to write the electron configuration of an atom, identify the energy level of. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide. Find the number of electrons in the atom: Alternatively, this can be abbreviated as [ar]4s²3d³, using the. [ar]4s 2 3d 3, 5. Web for the element vanadium, v, identify the electron configuration and the number of. Vanadium has an atomic number of 23, which means it has 23. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide. Web you simply have additional electrons to the neutral atom, and add them the same way you would for an atom. Web here's the. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web here's the electron configuration for vanadium: Alternatively, this can be abbreviated as [ar]4s²3d³, using the. First, write out the electron configuration for each parent atom. Web the electron configuration. Electrons always fill in the lowest energy configuration possible. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the periodic table as a guide. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web to find the electron configuration of oxygen: Note, although monatomic anions are isoelectronic to a nobel gas,. Find the number of electrons in the atom: Web the electron configuration of vanadium is [ar]3d34s2. Web here's the electron configuration for vanadium: The electron configuration for a neutral vanadium atom is 1s²2s²2p⁶3s²3p⁶4s²3d³. Read ratings & reviewsshop our huge selectionfast shippingshop best sellers Web the electronic configuration of vanadium can be represented as: Web what is the electron configuration of: List the subshells following the model shown in figure 1.6.3: Vanadium is defined as a chemical element belonging to the periodic table, it is part of group 5, it is represented by. Web what is the electron configuration and orbital diagram of: 1s2 2s2 2p6 3s2 3p6 3d3 4s2.
Draw the electron configuration for a neutral atom of vanadi Quizlet
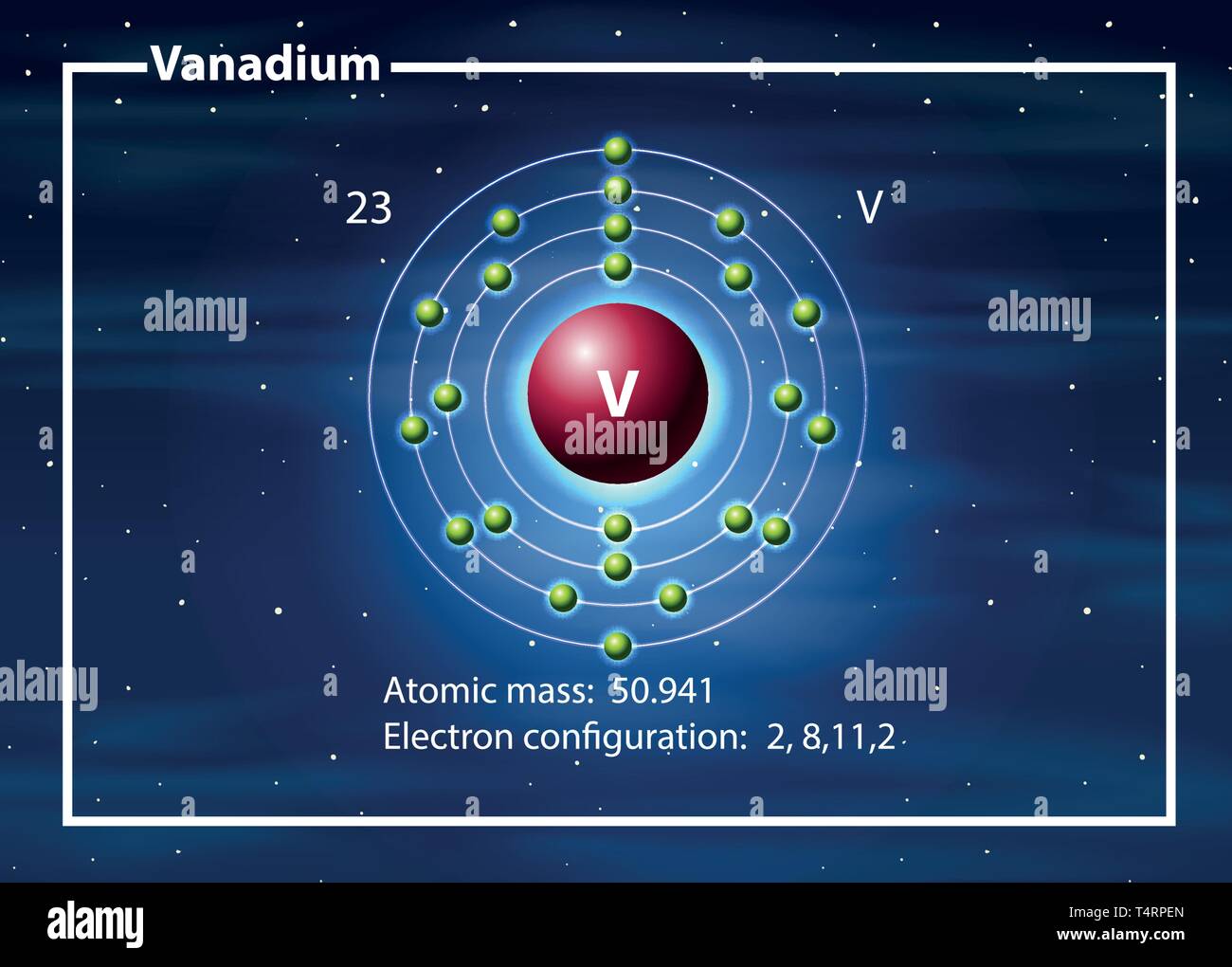
Draw The Electron Configuration For A Neutral Atom Of Vanadium. PIXMOB

Orbital Diagram For Vanadium (V) Vanadium Electron Configuration

Vanadium Facts, Symbol, Discovery, Properties, Uses
Solved Draw the electron configuration for a neutral atom of

Periodic Table Vanadium Periodic Table Timeline
SOLVED Draw the electron configuration for a neutral atom of vanadium
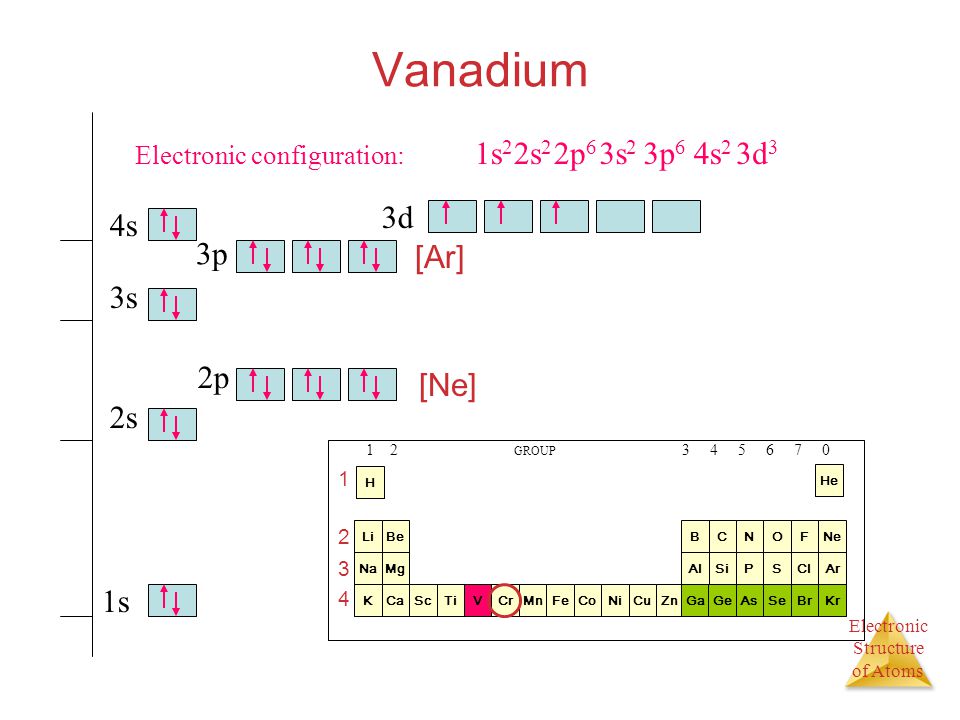
Vanadium Electron Configuration (V) with Orbital Diagram
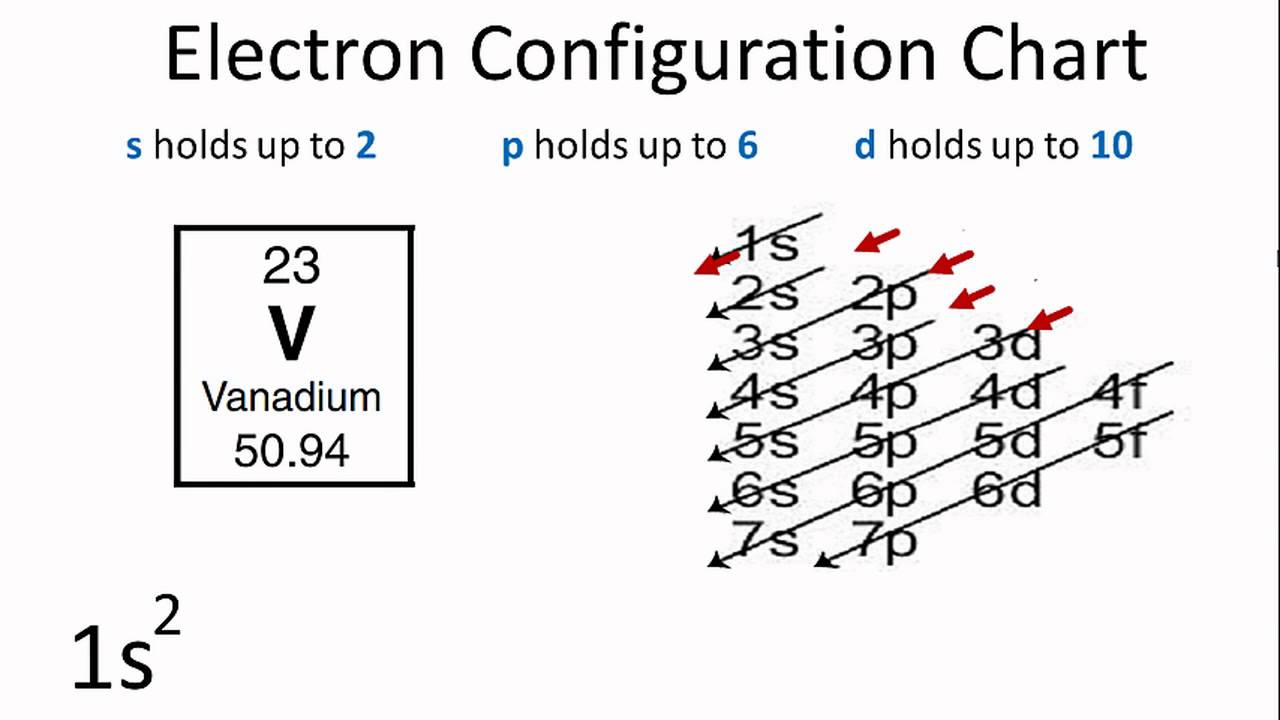
Vanadium Electron Configuration (V) with Orbital Diagram
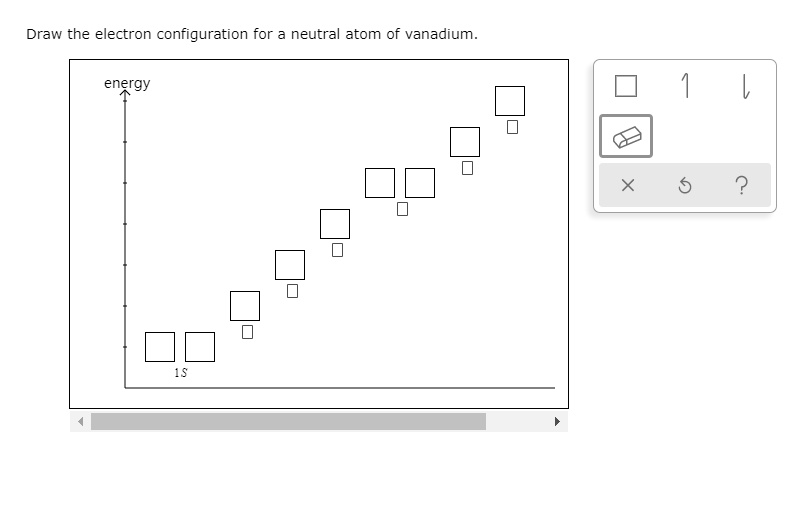
SOLVEDDraw the electron configuration for neutral atom of vanadium. energy
Aufbau Principle, Hund's Rule And The Pauli.
Web You Simply Have Additional Electrons To The Neutral Atom, And Add Them The Same Way You Would For An Atom.
Look At The Periodic Table And Find An Atomic Number Of Oxygen, Which Is 8.
Web Full Electron Configuration Of Vanadium:
Related Post: