Draw The Lewis Structure For Ch4
Draw The Lewis Structure For Ch4 - Web drawing the ch4 lewis structure. Determine the total number of valence electrons in the molecule. Web there is really only one way to draw the lewis structure for methane (ch4) which has only single bonds. One h atom is below the molecular plane and the other is above the. The final answer must have this number of electrons‼! Web the lewis structure of ch4 contains four single bonds, with carbon in the center, and four hydrogens on either side. Pcl 3 has 5 valence electros in p and 7 in each of the three cl: The molecule undergoes is sp3 hybridization. Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles. Web drawing the lewis structure for ch 4. Web drawing the ch4 lewis structure. = 4 + 1x4 = 8 valence electrons. C2h6 has a higher boiling point. There are following specifications in the lewis structure of methane. Web how to draw the lewis dot structure for ch4: For ch4, carbon has 4 valence electrons and hydrogen has 1 valence electron each. The final answer must have this number of electrons‼! There are no lone pairs in the valence shells of carbon atom. Web follow these simple steps to draw lewis dot structures: Calculate the total number of valence electrons. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web follow these simple steps to draw lewis dot structures: Web the ch4 molecule will have 109.5° bond angles as there is no distortion in its shape. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. We. Drawing the lewis structure for ch 4 (named methane) requires only single bonds.it's one of the easier lewis structures to draw. This is because they can share a maximum of two electrons. I also go over hybridization, shape and bond angle. 100% (1 rating) share share. A lewis structure can be drawn for any covalently bonded molecule, as well. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. I also go over hybridization, shape and bond angle. Be sure to have the correct number of electrons. This is because they can share a. The ch 4 lewis structure is one of the most frequently tested lewis structures. Finally, explain how you came to that conclusion. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons. Methane has four valence electrons from the carbon atom and one valence electron from each hydrogen atom, for a. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist. Web draw the lewis structure for ch4. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web drawing the lewis structure for ch 4. Methane has four valence electrons from the carbon atom and one valence electron from each hydrogen atom, for a. Find more chemistry widgets in wolfram|alpha. Be sure to have the correct number of electrons. There are no lone pairs on both carbon a. There are 2 steps to solve this one. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of ch4 is sp3. Once they have two valence electrons. Web check me out: 100% (1 rating) share share. Then predict which would have the higher boiling point. For ch 4 you have a total of 8 total valence electrons. It is the simplest hydrocarbon in the organic molecule and it is a hydride of c. Web the common name of ch4 is methane. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. There are 2 steps to solve this one. There are no lone pairs on both carbon a. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Drawing the ch4 lewis structure is a simple and straightforward process that can be done in a few easy steps. Following the steps, carbon is surrounded by 4 hydrogen atoms, and each hydrogen atom shares its electron with carbon. Determine the total number of valence electrons in the molecule. Web methane (ch 4) molecule lewis structure. Explains how to draw the lewis dot structure for ch4 (methane). A lewis structure can be drawn for any covalently bonded molecule, as well. The final answer must have this number of electrons‼! Web check me out: Web the lewis structure of the methane (ch4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Carbon atom is the center atom and it is very easy to draw ch4 lewis structure. This is because they can share a maximum of two electrons.
Lewis Structure CH4 plus dipoles, shape, angles and formal charge

Electron Dot Diagram For Water alternator

Draw Lewis Structure For Ch4 Nelson Tardwilis
Dot Diagram For Ch4

Lewis Dot Diagram Ch4

In this video we are going to learn about the Lewis structure of CH4

How To Draw The Lewis Structure For Ch4 Tanya Draw
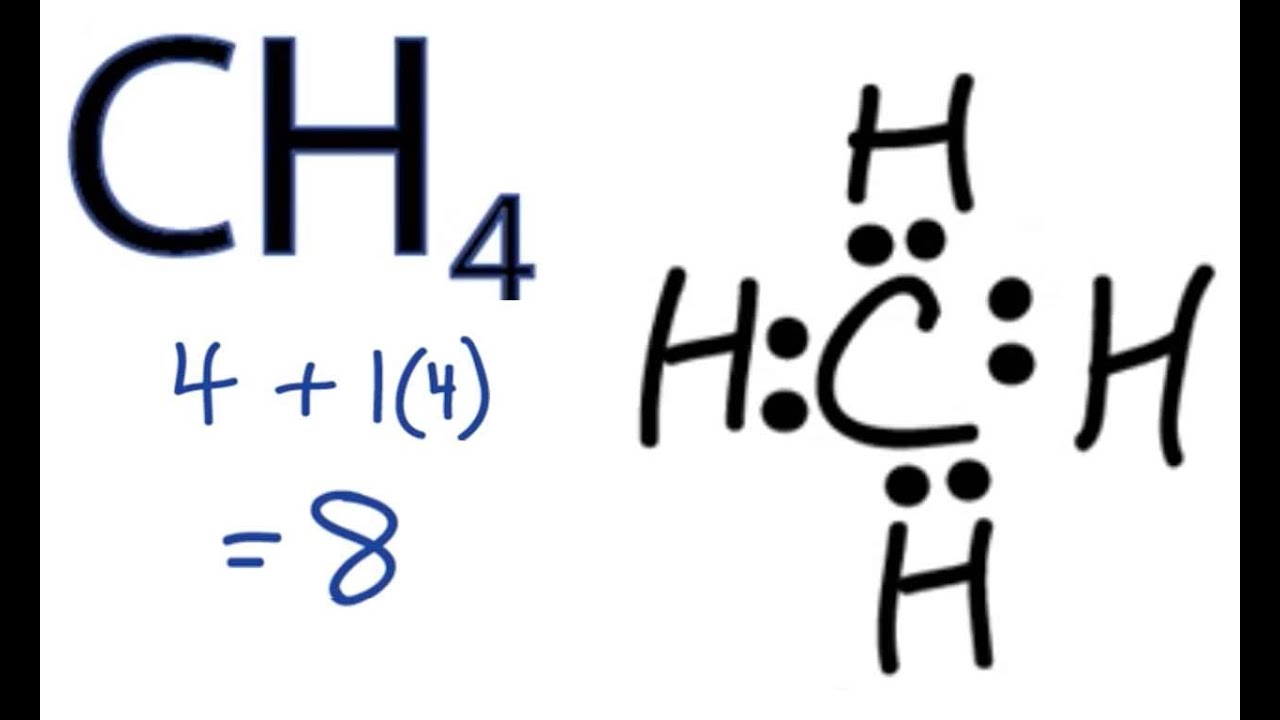
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane

CH4 Lewis Structure, Molecular Geometry, and Hybridization

How to Draw the Lewis Dot Structure for CH4 Methane YouTube
We Can Draw The Lewis Structure Of Any Covalent Molecule By Following The Six Steps Discussed Earlier.
It Is The Simplest Hydrocarbon In The Organic Molecule And It Is A Hydride Of C.
Generally, The Lone Pairs In The Molecule Distort The Shape Of The Molecule, Which Changes The Molecule’s Bond Angles.
One H Atom Is Below The Molecular Plane And The Other Is Above The.
Related Post: