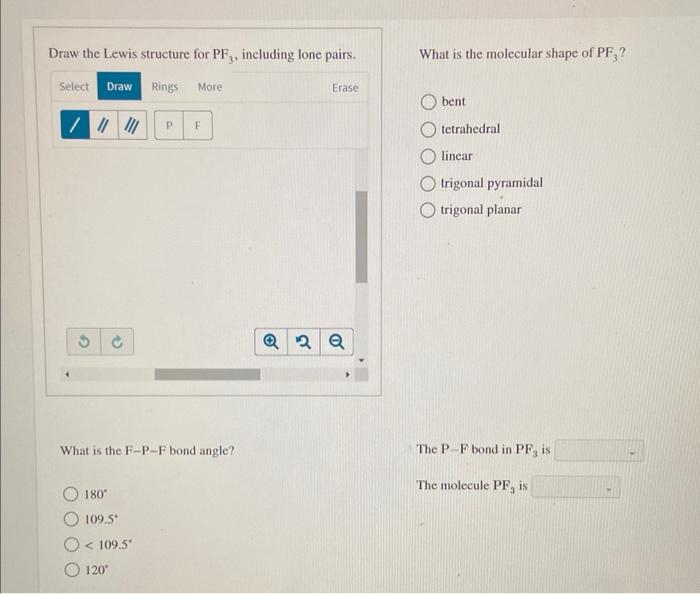
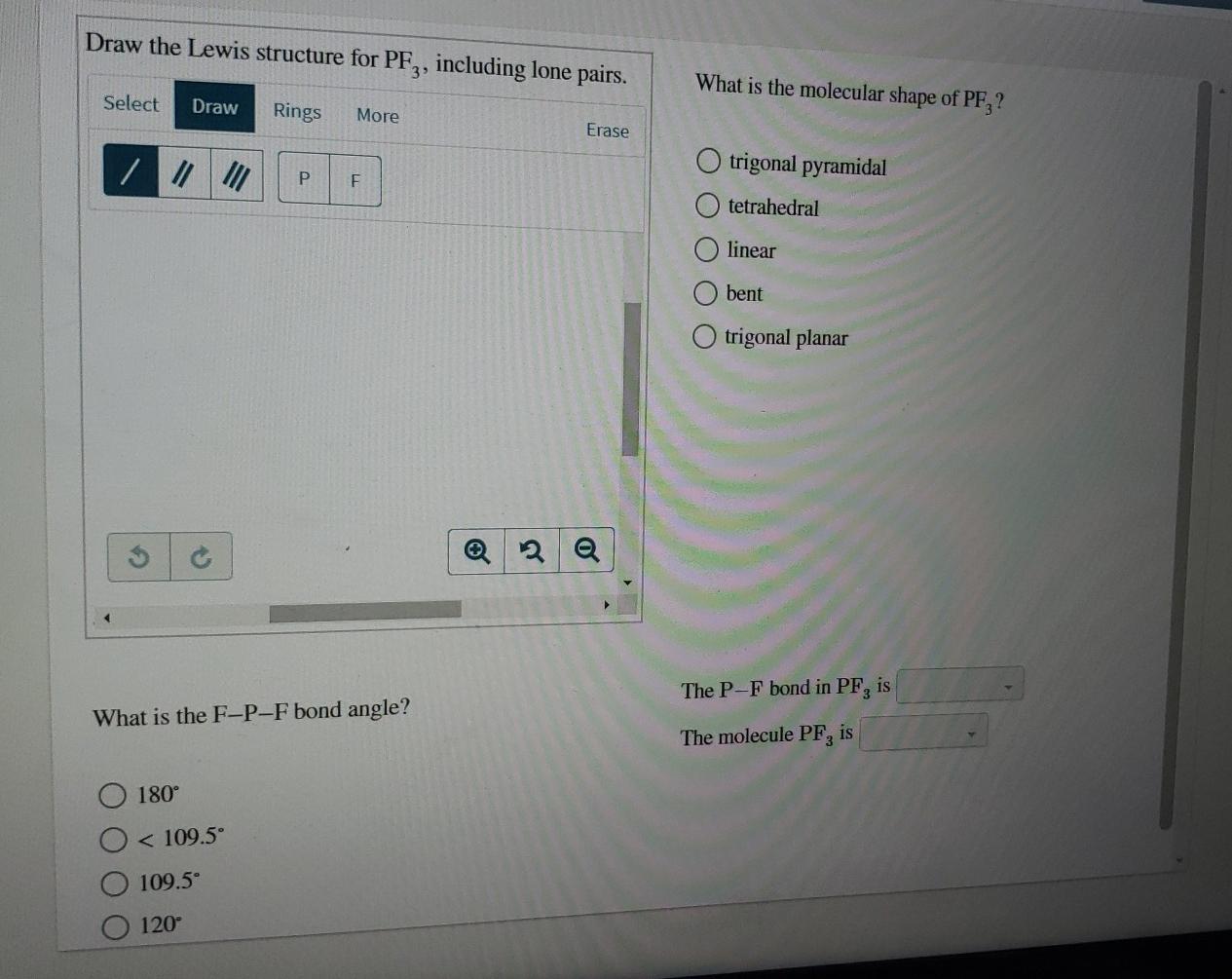
Draw The Lewis Structure For Pf3 Including Lone Pairs
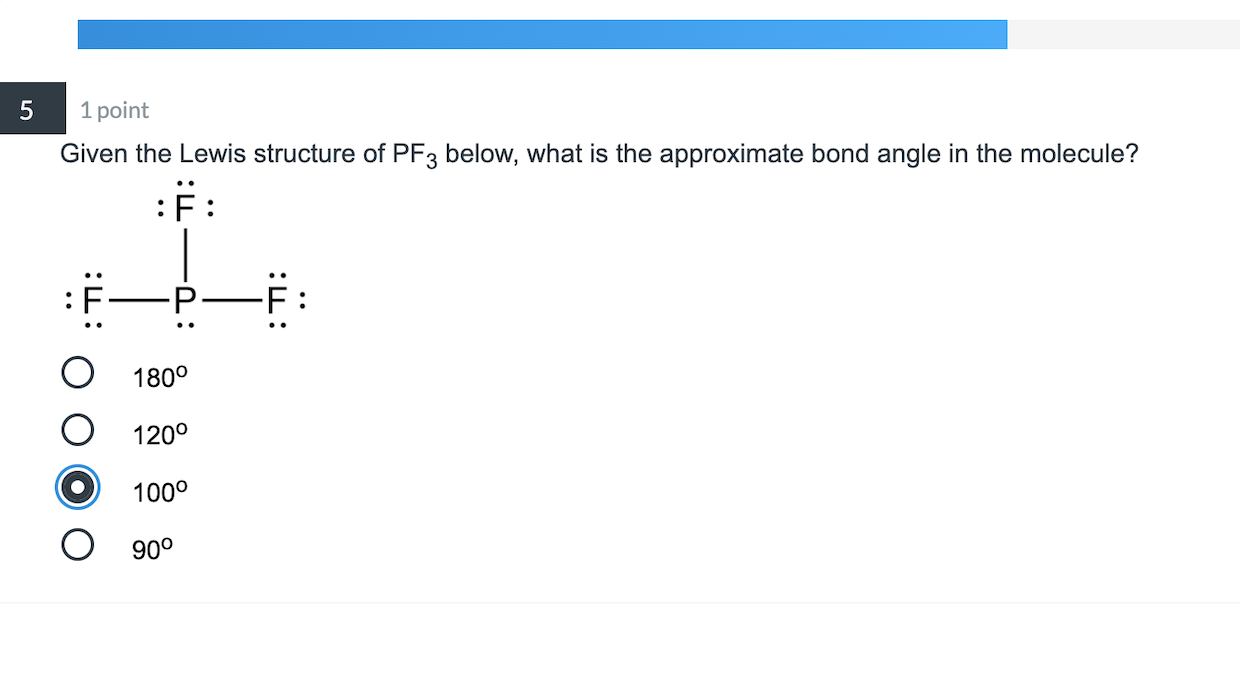
Draw The Lewis Structure For Pf3 Including Lone Pairs - What is the molecular shape of pf3 ? Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Pf3 lewis structure is made up of one phosphorous (p) atom located at the central position and three fluorine (f) atoms that. Web moving one lone pair from each terminal o atom, the following structure is obtained. Draw the lewis structure for pf3, including lone pairs. Web may 4, 2022 by biswarup chandra dey. If you realize, a single lone pair of electrons in. Write a lewis structure for the phosphorus trifluoride molecule, pf3. Web the lewis structure for pf3 shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron pairs. Answer a.3, 1 b.1, 3 c.3, 3 d.1, 2 e.2, 2. Back bonding occurs between the atoms, where one has a lone pair of electrons while the other has a vacant orbital. Draw the lewis structure for pf3, including all lone pair electrons and nonzero formal charges, and determine whether. Draw the lewis structure for pf3, including lone pairs. Web moving one lone pair from each terminal o atom, the following. Web how to draw lewis structure of pf3. Web the o has two bonding pairs and two lone pairs, and c has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs). Pf3 lewis structure is made up of one phosphorous (p) atom located at the central position and three fluorine (f) atoms that. Web how to draw lewis structure of pf3. Draw a lewis structure for each of the following compounds. Include all lone pair electrons. If you realize, a single lone pair of electrons in. Draw the lewis dot structure for pf3. Web valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Web the o has two bonding pairs and two lone pairs, and c has four bonding pairs. If you realize, a single lone pair of electrons in. Web may. Web the o has two bonding pairs and two lone pairs, and c has four bonding pairs. If you realize, a single lone pair of electrons in. Draw a lewis structure for each of the following compounds. So this appears to be the first answer option there. This article discusses pf3 lewis structure and its hybridization, shape, bond angle, and. Back bonding occurs between the atoms, where one has a lone pair of electrons while the other has a vacant orbital. This is the complete lewis structure of co 2. Web the lewis structure for pf3 shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron pairs. Answer a.3, 1 b.1, 3 c.3, 3 d.1, 2 e.2,. Web alright, so we have our lewis structure and we determined the hybridization is sp three d two and the molecular geometry is a square planer. This is the structure of formaldehyde, which is used in embalming fluid. Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f) atom. Draw the lewis. Web how to draw lewis structure of pf3. Answer a.3, 1 b.1, 3 c.3, 3 d.1, 2 e.2, 2. Pf3 lewis structure is made up of one phosphorous (p) atom located at the central position and three fluorine (f) atoms that. Web the lewis structure for pf3 shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron. Web how to draw lewis structure of pf3. What is the molecular shape of pf3 ? Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Include all lone pair electrons. Web may 4, 2022 by biswarup chandra dey. This article discusses pf3 lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. Web alright, so we have our lewis structure and we determined the hybridization is sp three d two and the molecular geometry is a square planer. Web lewis structure of pf3 contains three single bonds between the phosphorus (p) atom and each fluorine (f). Web in the pf 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. Web the lewis structure for pf3 shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron pairs. Web how to draw lewis structure of pf3. Web valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Pf3 lewis structure is made up of one phosphorous (p) atom located at the central position and three fluorine (f) atoms that. This article discusses pf3 lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. This is the complete lewis structure of co 2. Web may 4, 2022 by biswarup chandra dey. Web in the pf 3 lewis structure, there are three single bonds around the phosphorus atom, with three fluorine atoms attached to it. Draw the lewis structure for pf3, including lone pairs. What is the molecular shape of pf3 ? Draw the lewis structure for pf3, including lone pairs. Answer a.3, 1 b.1, 3 c.3, 3 d.1, 2 e.2, 2. What is the molecular shape of pf3 ? The phosphorus atom (p) is at the center and it is. Include all lone pair electrons.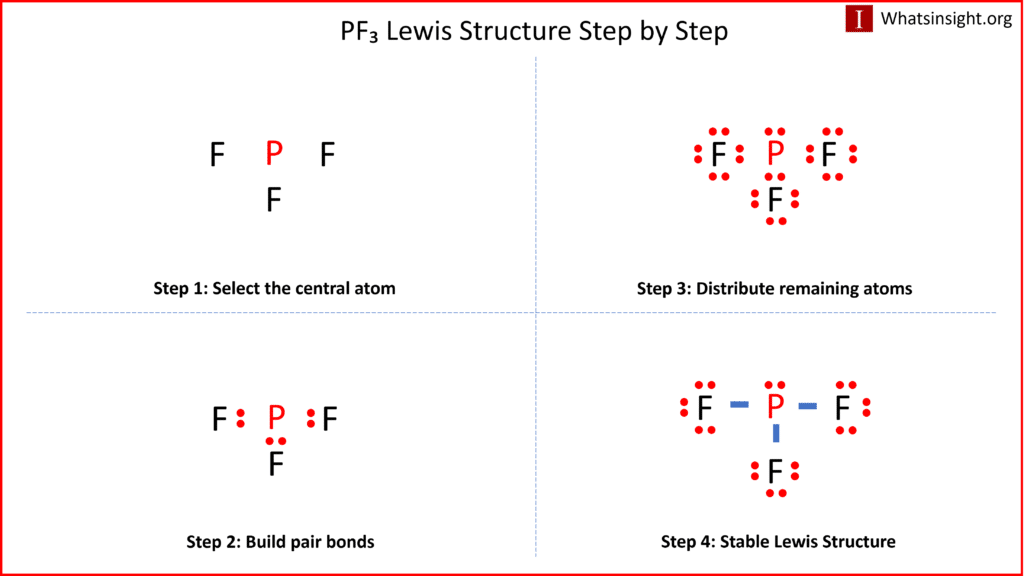
PF3 Lewis structure in four simple steps What's Insight
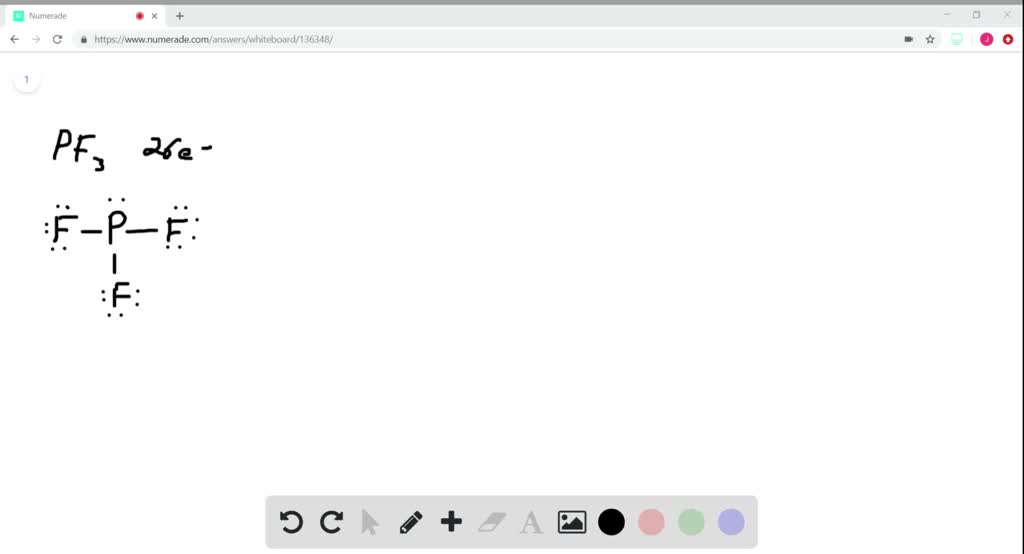
Pf3 Lewis Structure

How to draw PF3 Lewis Structure? 2
Solved Draw the Lewis structure for PF3, including lone
Lewis Structure For Pf3 Drawing Easy
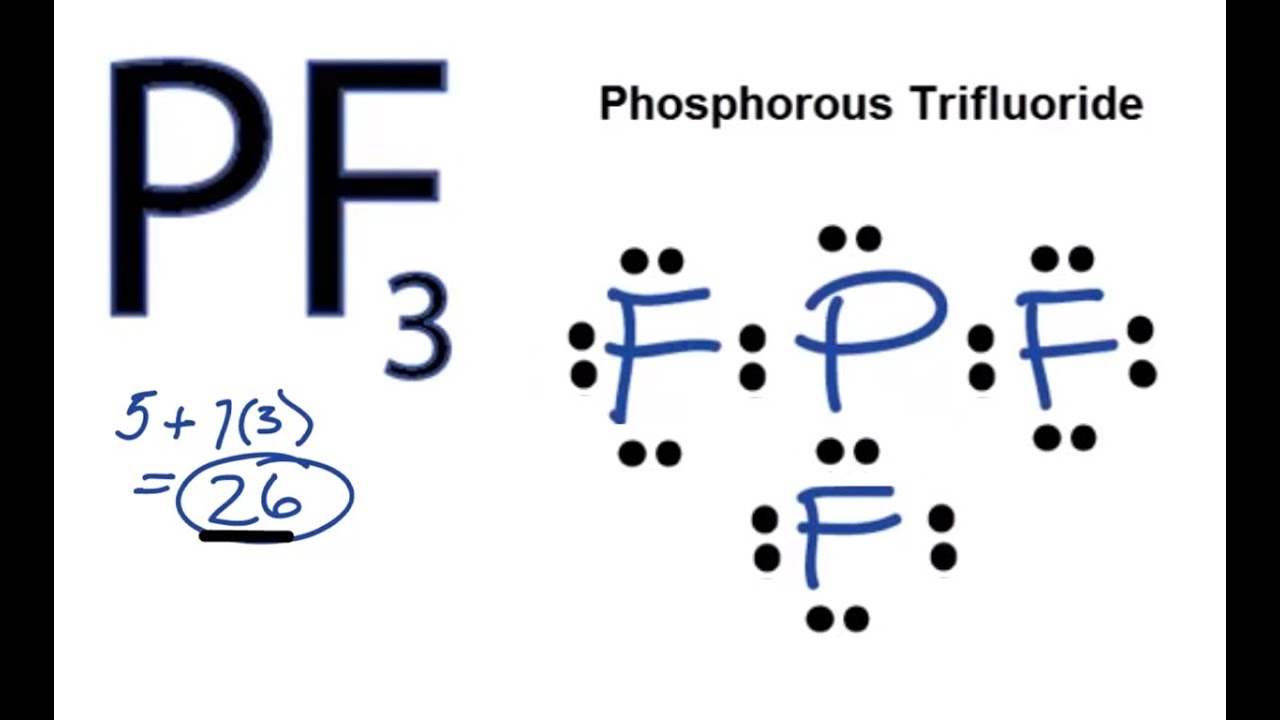
PF3 Lewis Structure How to Draw the Lewis Structure for PF3 YouTube

Pf3 Lewis Structure Molecular Geometry
[Solved] Draw the Lewis structure for PF3 on your shown work (P is the

draw the lewis structure for pf3 including lone pairs 160vanbruntstreet
draw the lewis structure for pf3 including lone pairs bettinaniedermaier
Web Lewis Structure Of Pf3 Contains Three Single Bonds Between The Phosphorus (P) Atom And Each Fluorine (F) Atom.
In The Lewis Structure For Pf 3 There Are A Total Of 26 Valence Electrons.
Web Alright, So We Have Our Lewis Structure And We Determined The Hybridization Is Sp Three D Two And The Molecular Geometry Is A Square Planer.
Web The O Has Two Bonding Pairs And Two Lone Pairs, And C Has Four Bonding Pairs.
Related Post: