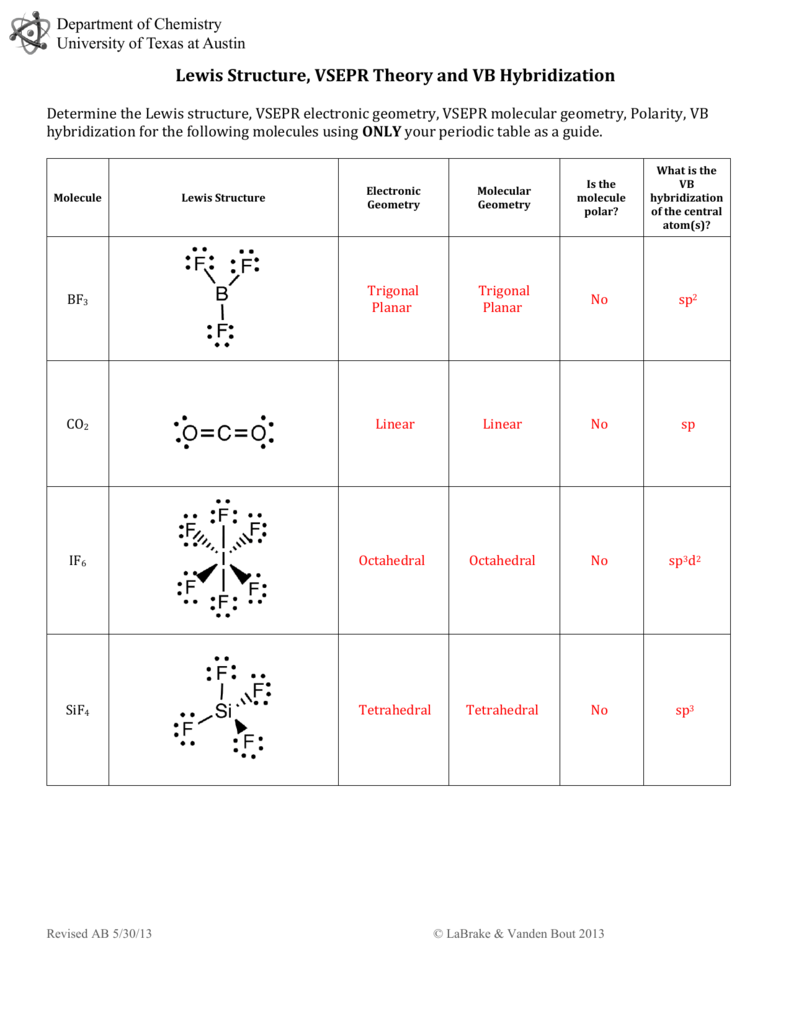
Draw The Lewis Structure For Sif4
Draw The Lewis Structure For Sif4 - To draw the lewis structure of the molecules. Web where needed, rearrange electrons to form multiple bonds in order to obtain an octet on each atom: The video also discusses the molecular geometry, bond angle, and if sif4 is polar or nonpolar. You do not need to include nonzero formal charges in your structures. Web include all lone pair electrons in your structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw a lewis structure for the following. Determine the number of valence electrons available for the molecule sif4. Draw the lewis structure for sif4; Gef4 behaves similarly, but cf4 does not. Web sif4 lewis structure shape; (a) draw lewis structures for the following species, making sure to show any lone pairs of electrons. The shape of a molecule depends upon the repulsion between the valence electron bond pair or nonbonding pair. In the sif4 molecule, the four fluorine atoms are surrounded by a central silicon atom. Here’s the best way to. Formal charge(n) = 5 − (0 + 8 2) = 0. Find the total valence electrons in sif4 molecule. Tructure in the space below an atom. In the sif4 molecule, the four fluorine atoms are surrounded by a central silicon atom. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons. (c) cof2 (c is central) The sif4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. The video also discusses the molecular geometry, bond angle, and if sif4 is polar or nonpolar. Tructure in the space below an atom. (valence electrons are the number of electrons present in the outermost shell of an. 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. Draw a lewis structure for (a) sif4; 4 + (3 × 6) + 2 = 24 electrons. Gef4 behaves similarly, but cf4 does not. Using equation 8.5.1, the formal charge on the nitrogen atom is therefore. The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Web how to draw lewis structure for sif4 silicon tetrafluoridelewis structure: (c) cof2 (c is central) here’s the best way to solve it. Form a triple bond between the two carbon atoms. Tructure in the space below an atom. Web in the sif4 lewis structure, there are four single bonds around the silicon atom, with four fluorine atoms attached to it, and on each fluorine atom, there. (valence electrons are the number of electrons present in the outermost shell of an atom). Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3). 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. You do not need to include nonzero formal charges in your structures. Web the lewis electron structure for the nh 4+ ion is as follows: Let us draw the lewis structure of silicon tetrafluoride. Web this problem has been solved! Find out the total number of valence electrons in silicon tetrafluoride. It will be necessary to draw and. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Draw a lewis structure for (a) sif4; Web include all lone pair electrons. (c) cof2 (c is central) You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The sif4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. In order to draw the lewis structure of sif4, first of all you have to find the total number of valence. The final answer must have this number of electrons‼! Determine the number of valence electrons available for the molecule sif4. All atoms have the correct number of electrons. Web (a) silicon tetrafluoride (sif4) reacts with f− to produce the hexafluorosilicate ion, sif62−. (valence electrons are the number of electrons present in the outermost shell of an atom). Formal charge(n) = 5 − (0 + 8 2) = 0. The sif4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. (c) cof2 (c is central) here’s the best way to solve it. Form a triple bond between the two carbon atoms. #1 draw a rough sketch of the structure #2 next,. Include all lone pair electrons in your structure. Draw the lewis structure for sif4; Let us draw the lewis structure of silicon tetrafluoride. Web draw the molecule by placing atoms. Draw lewis structure for each of the compounds and ions listed below. Web sif4 lewis structure shape; Find out the total number of valence electrons in silicon tetrafluoride. Tructure in the space below an atom. Web include all lone pair electrons in your structure. In the sif4 molecule, the four fluorine atoms are surrounded by a central silicon atom. We're going to do the lewis structure for sif4.
Lewis Structure Of Sif4
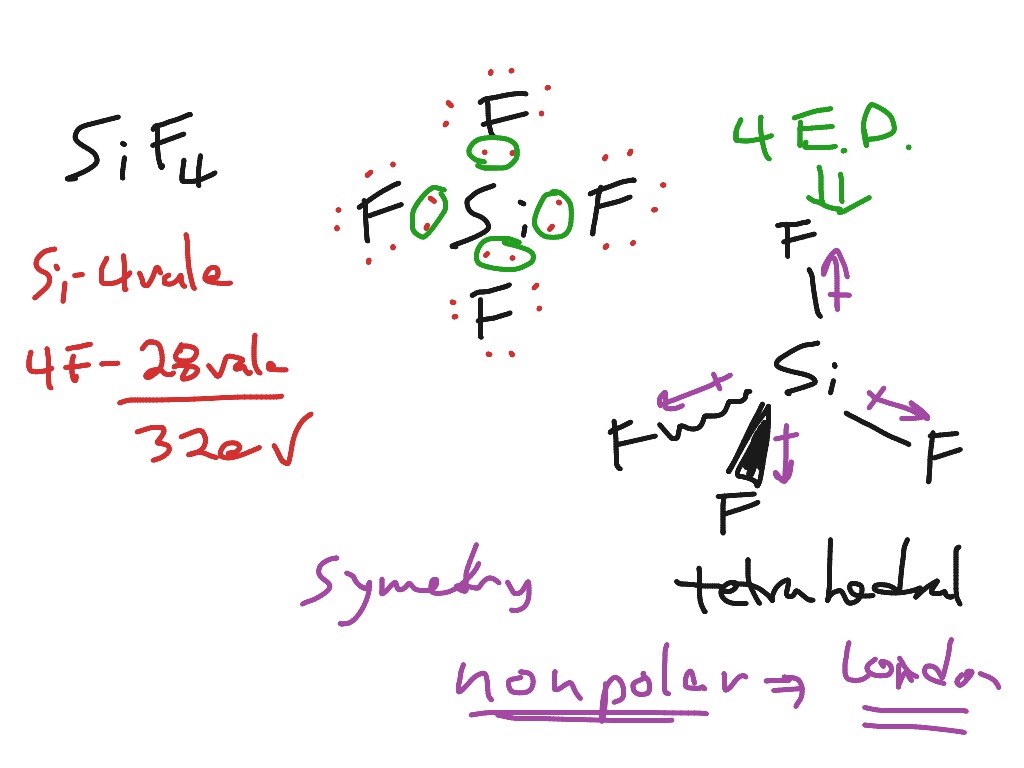
Sif4 Lewis Structure
Draw the Lewis structure for SiF4; arrangement of ato… SolvedLib

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Sif4 Lewis Structure

Lewis Structure For Sif4
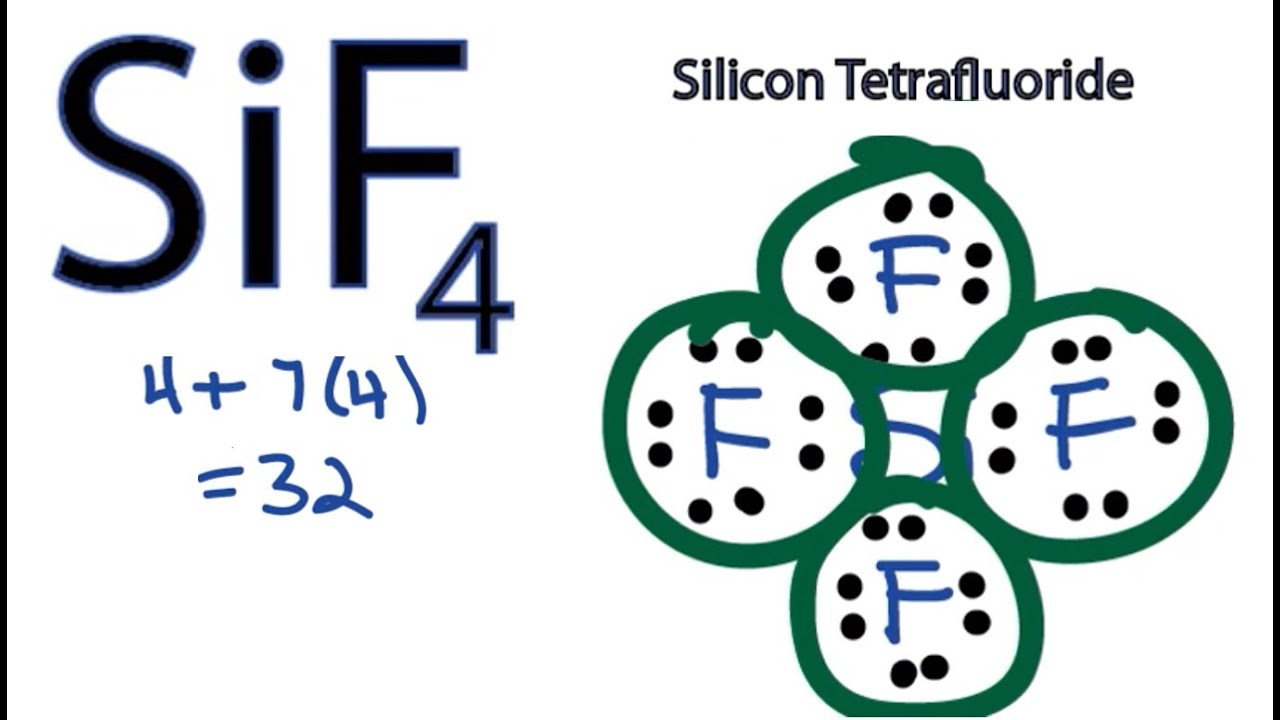
SiF4 Lewis Structure How to Draw the Dot Structure for SiF4 YouTube
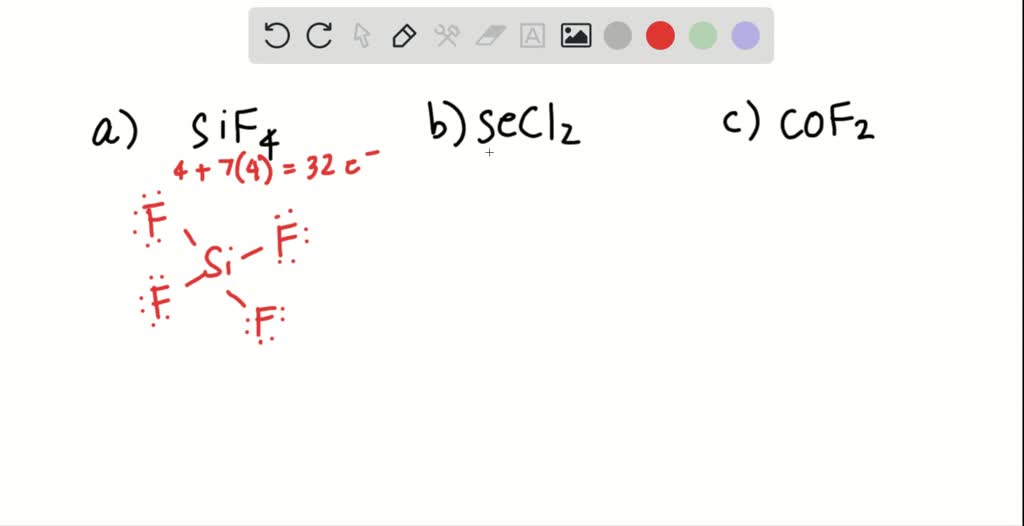
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is
Lewis Structure Of Sif4

Sif4 Lewis Structure
How To Draw Lewis Structure For Sif4.
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As Dots Next To Atoms.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Draw A Lewis Structure For (A) Sif4;
Related Post: