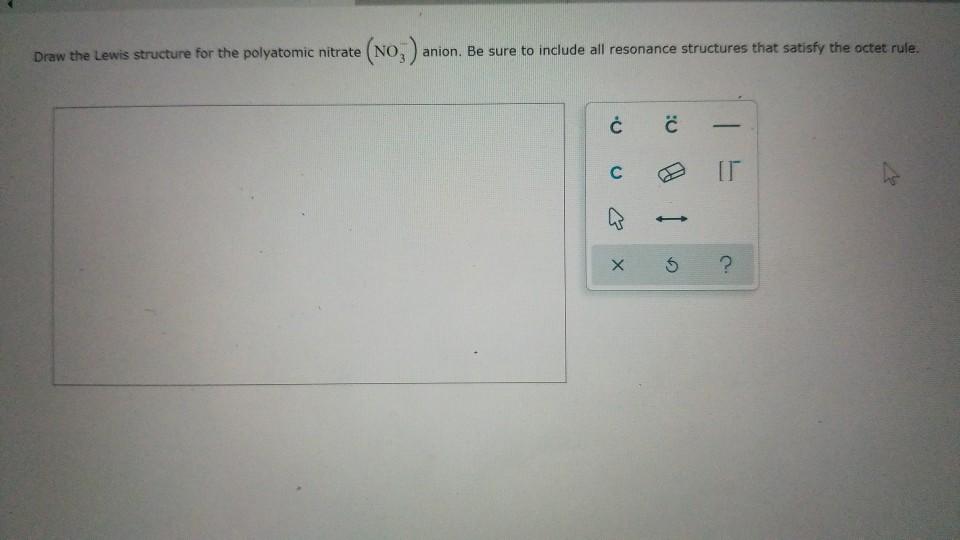
Draw The Lewis Structure For The Polyatomic Nitrate
Draw The Lewis Structure For The Polyatomic Nitrate - Web resonance arises when more than one valid lewis structure can be drawn for a molecule or ion. Web in this video, we will go through how to draw lewis structures for polyatomic ion in five easy steps. Web draw the lewis structure for the polyatomic nitrate anion. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Assign formal charge to each atom in the lewis structure. Be sure to include all resonance structures that satisfy the octet rule. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to as the resonance hybrid. Web a simple short video showing how to draw the lewis structure of the polyatomic ion nitrate (nitrate) Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. Here’s the best way to solve it. Assign formal charge to each atom in the lewis structure. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This problem has been solved! Be sure to include all resonance structures that satisfy the octet rule. That will tell us which atoms are sharing electrons. Be sure to include all resonance structures that satisfy the octet rule. Web often you need to decide how the atoms are arranged before writing the lewis structure. Here’s the best way to solve it. Web this widget gets the lewis structure of chemical compounds. Web in this video, we will go through how to draw lewis structures for polyatomic. Web draw the lewis structure for the polyatomic nitrate (no3−)anion. Web draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. Be sure to include all resonance structures that satisfy the octet rule. Web lewis dot structure of polyatomic ions. Web resonance structures are a set of two or more lewis. That will tell us which atoms are sharing electrons. Web draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. Web draw the lewis structure for the polyatomic nitrite (no2) anion. Be sure to include all resonance structures that satisfy the octet rule. Web often you need to decide how the. At least two lewis structures can be drawn for bcl 3. Finally, draw two additional resonance structures; Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. Web this widget gets the lewis structure of chemical compounds. Web in chem 101a,. A) draw the lewis structure for the nitrite polyatomic anion and indicate the electron geometry & molecular geometry, the hybridization around the central atom, and the number of sigma and pi bonds in the polyatomic ion. Here’s the best way to solve it. Web often you need to decide how the atoms are arranged before writing the lewis structure. Web. Using arguments based on formal charges, explain why the most likely. Web lewis dot structure of polyatomic ions. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web this widget gets the lewis structure of chemical compounds. Web draw the lewis structure for the polyatomic nitrate no−3 anion. At least two lewis structures can be drawn for bcl 3. The exceptions to octet rule will be. Web this widget gets the lewis structure of chemical compounds. Be sure to include all resonance structures that satisfy the octet rule. Web resonance arises when more than one valid lewis structure can be drawn for a molecule or ion. We could put one atom in the middle of the structure and arrange all the others around it. Be certain to include formal charges on resonance structures as well. Finally, draw two additional resonance structures; This problem has been solved! How to draw lewis structures. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw the lewis structure for the polyatomic nitrite (no2) anion. Form single bonds between nitrogen and each oxygen atom, fill the remaining electrons, and check for incomplete octets. Let's draw the structure for carbonic acid, h 2 co 3. This problem has been solved! Web there are three lewis structures that you can draw for c2h2f2. Form single bonds between nitrogen and each oxygen atom, fill the remaining electrons, and check for incomplete octets. There are 2 steps to solve this one. Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. Be certain to include formal charges on resonance structures as well. Let's draw the structure for carbonic acid, h 2 co 3. The exceptions to octet rule will be. Web draw the lewis structure for the polyatomic nitrite (no2) anion. We could put one atom in the middle of the structure and arrange all the others around it. Find more chemistry widgets in wolfram|alpha. How to draw lewis structures. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to as the resonance hybrid. Be sure to include all resonance structures that satisfy the octet rule. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Be sure to include all resonance structures that satisfy the octet rule. Assign formal charge to each atom in the lewis structure.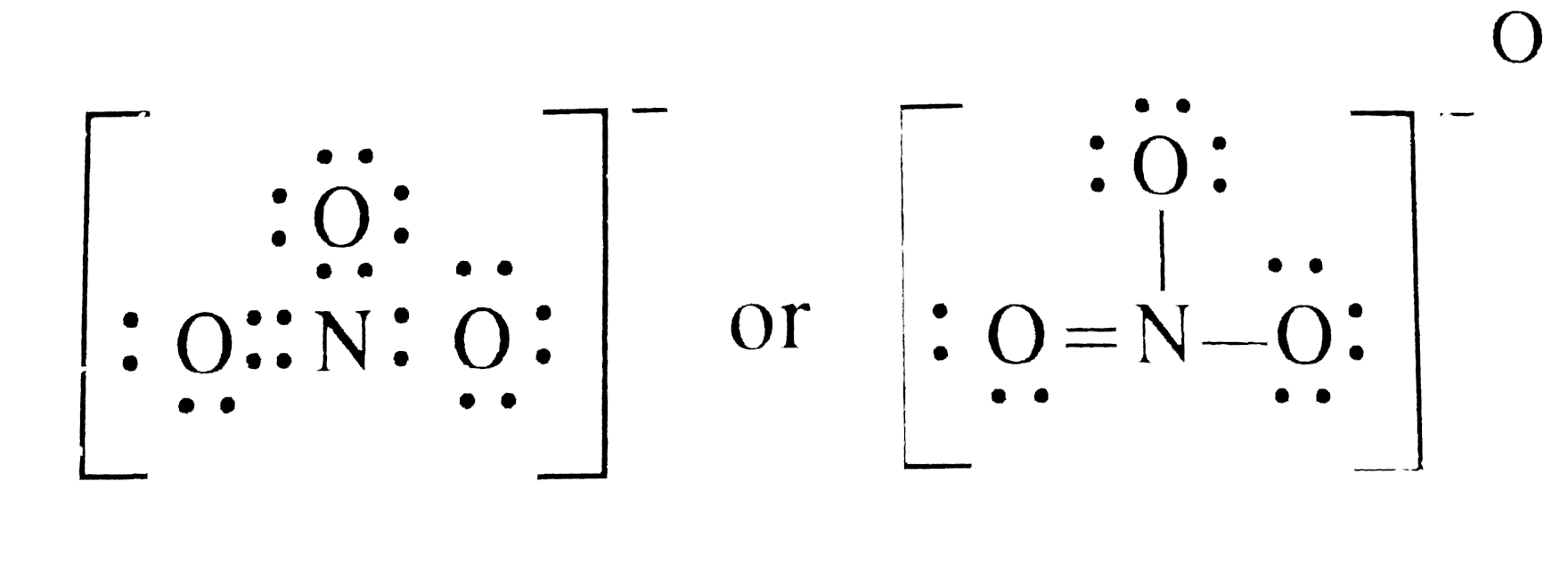
Write the various steps involved in the Lewis structure for nitrate (N

What are Polyatomic Ions? Give Examples Teachoo Concepts
[Solved] Consider the polyatomic ion nitrate, NO3 . Draw the Lewis
Solved Draw a Lewis Structure for the nitrate polyatomic ion
SOLVED Draw the Lewis structure for the polyatomic nitrate anion. Be
[Solved] Draw all valid Lewis structures for the nitrate ion, showing
[Solved] Consider the polyatomic ion nitrate, NO3 . Draw the Lewis
Solved Draw the Lewis structure for the polyatomic nitrate
Solved Draw the Lewis structure for the polyatomic nitrate

Nitrate Ion Lewis Structure How to Draw the Lewis Structure for
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Using Arguments Based On Formal Charges, Explain Why The Most Likely.
A) Draw The Lewis Structure For The Nitrite Polyatomic Anion And Indicate The Electron Geometry & Molecular Geometry, The Hybridization Around The Central Atom, And The Number Of Sigma And Pi Bonds In The Polyatomic Ion.
Web In Chem 101A, We Will Focus On Drawing Lewis Structures Of Molecules And Polyatomic Ions That Have One Central Atom With Several Other Atoms Attached To It.
Related Post: