Draw The Lewis Structure Of N2H2
Draw The Lewis Structure Of N2H2 - Does h have a duet of electrons? Web how to draw the lewis dot structure for n2h2: The molecule is made up of two hydrogen atoms and two nitrogen atoms. There are two nitrogen atoms and two hydrogen atoms in n2h2, giving us a total of 12 valence electrons. Hydrogen (h) only needs two valence electrons to have a full outer shell. N 2 h 2 is straightforward with no double or triple bonds. Draw the molecule by placing atoms on the grid and connecting them with bonds. Find the total valence electrons in n2h2 molecule. There are 4 steps to solve this one. Draw the lewis structure of diazine, n_2h_2. #3 indicate formal charges on the atoms, if necessary. Draw the lewis structures of n_2h_4, n_2h_2, and n_2. Draw a lewis structure for each species. Lewis structure has a lot of drawbacks even though it is one of the foremost and simplest methods to discuss chemical bonding. • draw one structure per sketcher box, and separate added sketcher boxes with. Does n have an octet? In appearance it is a yellow colored gas. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Added jun 9, 2014 by webtester in chemistry. Include all lone pairs of electrons. Determine the total number of valence electrons in the molecule by adding the valence electrons of all the atoms in the molecule. #5 repeat step 4 if necessary, until all charges are minimized. What is the hybridization on the n atoms? Draw a lewis structure for each species. • draw one structure per sketcher box, and separate added sketcher boxes. Include all lone pairs of electrons. N 2 h 2 is straightforward with no double or triple bonds. Lewis structure has a lot of drawbacks even though it is one of the foremost and simplest methods to discuss chemical bonding. Draw the molecules by placing atoms on the grid and connecting them with bonds. Web draw the lewis structure for. Draw the lewis structure of diazine, n_2h_2. In the n 2 h 2 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). For the n2h2 structure use the periodic table to find the total number of valence electrons for the. In appearance it is a yellow colored gas. #5 calculate formal charge. Here, the given molecule is n2h2 (or dinitrogen dihydride). We will first learn the lewis structure of this molecule to understand its physical and chemical properties better. Include all lone pairs of electrons. N 2 h 2 is straightforward with no double or triple bonds. Figure out how many electrons the molecule must have, based on the number of valence. Web to properly draw the n 2 h 2 lewis structure, follow these steps: Steps of drawing n2h2 lewis structure. There are 4 steps to solve this one. What is the hybridization on the n atoms? Include all lone pairs of electrons and hydrogen atoms. Draw the molecule by placing atoms on the grid and connecting them with bonds. How many total electrons do you have available? Web drawing lewis structures for molecules with one central atom: This video solution was recommended by our tutors as helpful for the problem above. Nitrogen (n) has five valence electrons, while hydrogen (h) has one valence electron. For the n2h2 structure use the periodic table to find the. Nitrogen (n) has five valence electrons, while hydrogen (h) has one valence electron. #4 minimize formal charges by converting lone pairs of the atoms. Calculate the total number of valence electrons. In order to draw the lewis structure of n2h2, first of all you have to find the total. 96k views 10 years ago. Hch2 367 carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized. Draw the molecule by placing atoms on the grid and connecting them with bonds. Does h have a duet of electrons? Find the total valence electrons in n2h2 molecule. N 2 h 2 is straightforward with no double or triple bonds. Draw the lewis structures of n_2h_4, n_2h_2, and n_2. Added jun 9, 2014 by webtester in chemistry. Determine the total number of valence electrons in the molecule by adding the valence electrons of all the atoms in the molecule. #4 minimize formal charges by converting lone pairs of the atoms. Hch2 367 carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized. Web the structure of n2h2 consists of 2 atoms each of nitrogen and oxygen. In the n 2 h 2 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). Find the total valence electrons in n2h2 molecule. Draw the molecules by placing atoms on the grid and connecting them with bonds. For the n2h2 structure use the periodic table to find the. Draw the molecule by placing atoms on the grid and connecting them with bonds. For the n2h2 structure use the periodic table to find the total number of valence electrons for the. Thus far, we have discussed the lewis structure of atoms and ionic compounds. Web 6 steps to draw the lewis structure of n2h2 step #1: Draw a lewis structure for each species.
N2h2 Lewis Structure Molecular Geometry Draw Easy

N2H2 Lewis Structure Lewis Dot Structure for N2H2 Dinitrogen

How to Draw the Lewis Dot Structure for N2H2 Diazene YouTube
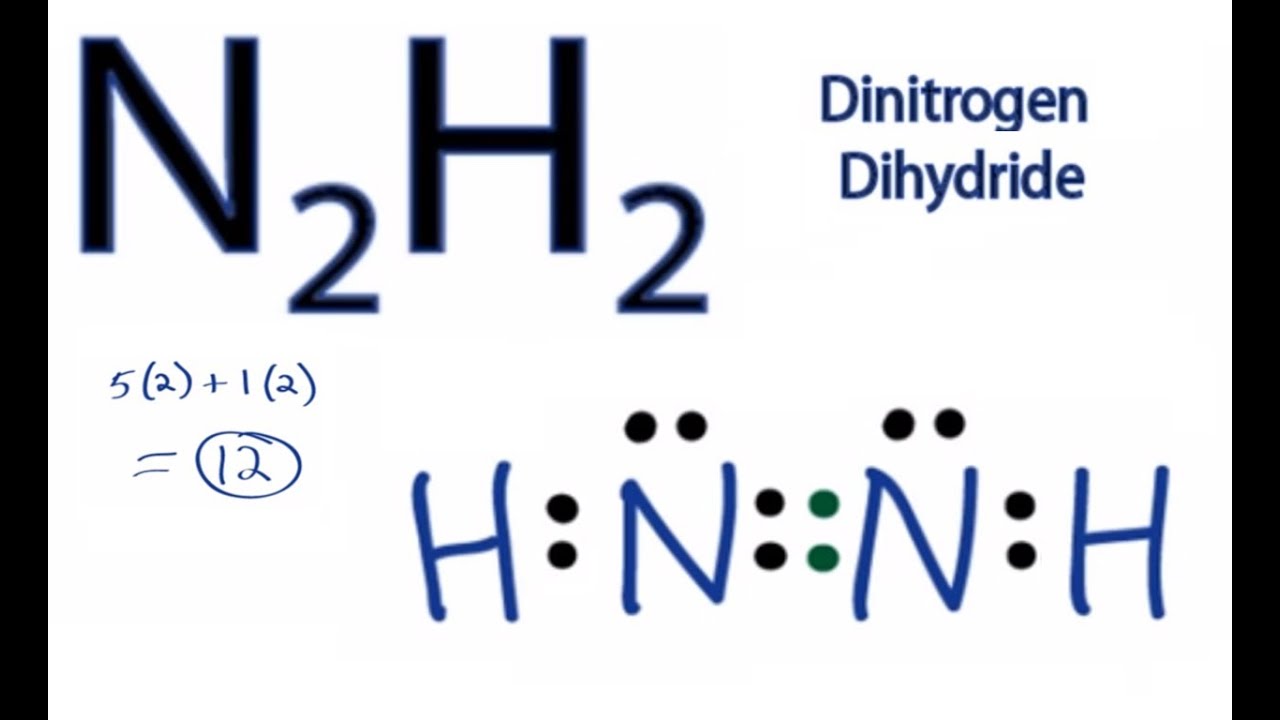
N2H2 Lewis Structure How to Draw the Lewis Structure for Dinitrogen

N2h2 Lewis Structure Molecular Geometry Draw Easy

The Correct Lewis Structure Of N2h2 Shows Draw Easy

N2H2 Lewis Structure & Characteristics (13 Helpful Facts) LAMBDAGEEKS
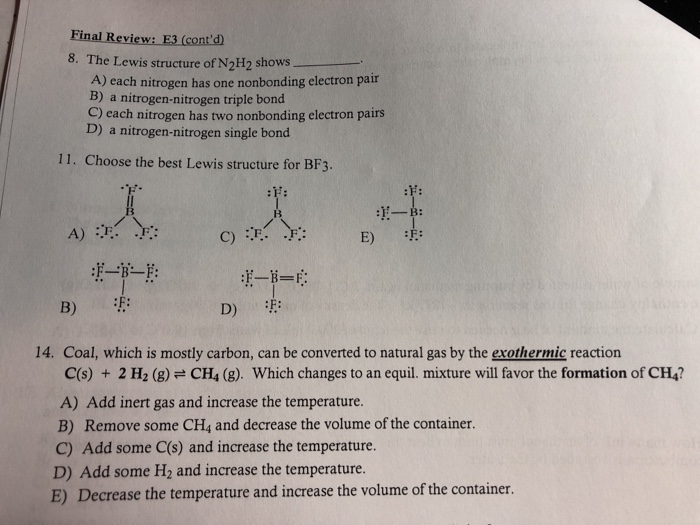
The Correct Lewis Structure Of N2h2 Shows Draw Easy

N2H2 Lewis Structure (Dinitrogen Dihydride) YouTube
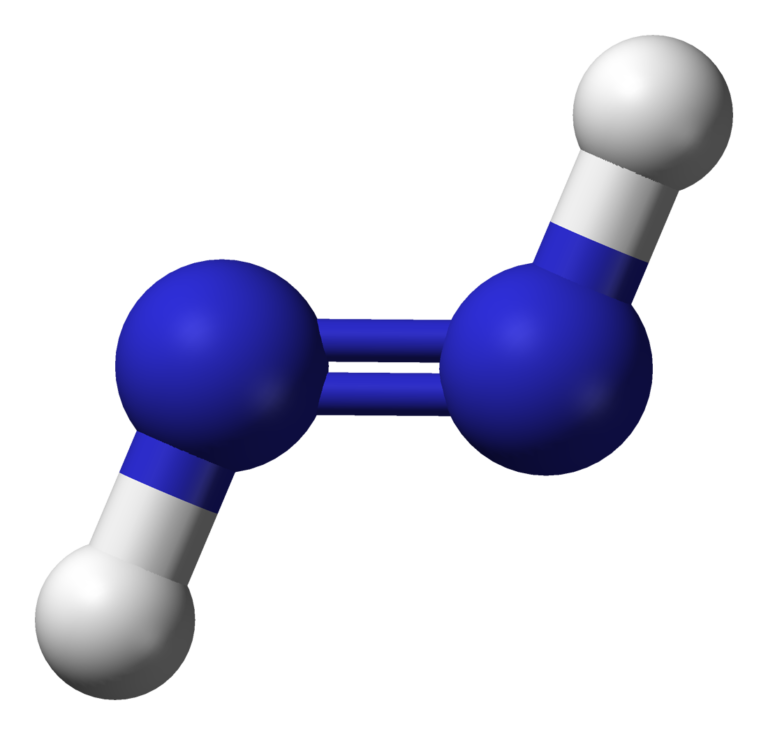
N2H2 Lewis structure, Molecular Geometry, Hybridization, Bond Angle and
Draw Lewis Structures For The Hydrazine Molecule ( N2H4), The Nitrogen Molecule (N2), And The Diazene Molecule (N2H2), And Then Answer The Questions That Follow.
Now, We Have Got The Most Suitable Lewis Structure For N2H2.
This Widget Gets The Lewis Structure Of Chemical Compounds.
The Following Procedure Will Give You The Correct Lewis Structure For Any Molecule Or Polyatomic Ion That Has One Central Atom.
Related Post: