High Specific Heat Drawing
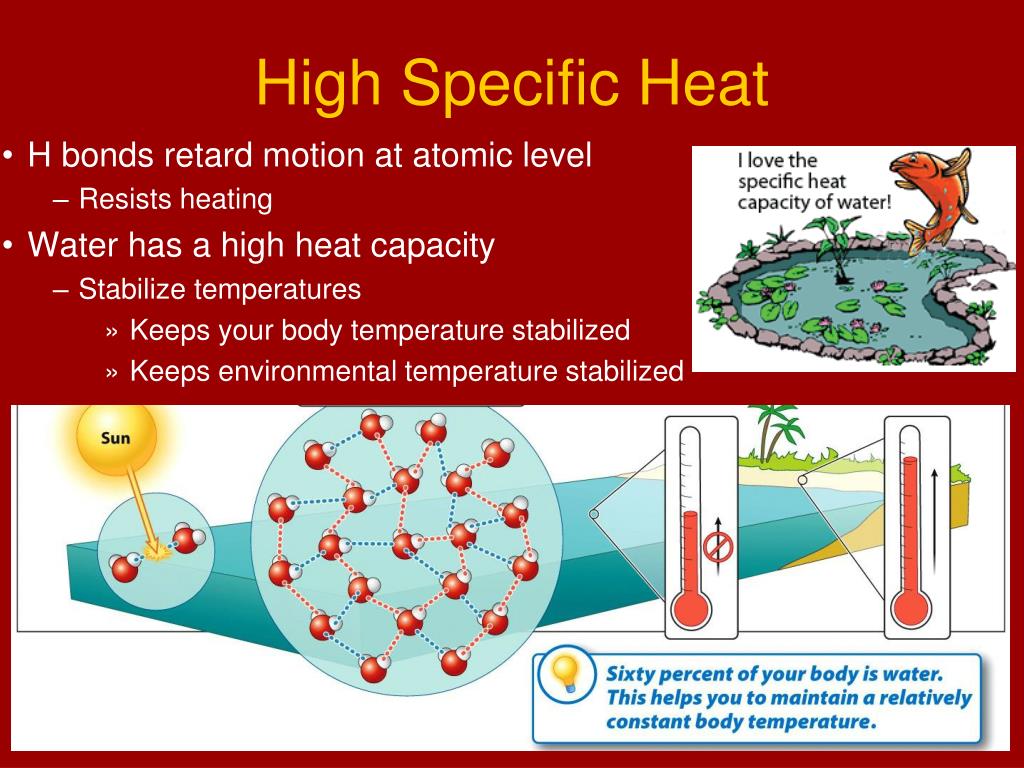
High Specific Heat Drawing - How to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. Web in other words, water has a high specific heat capacity, which is defined as the amount of heat needed to raise the temperature of one gram of a substance by one degree celsius. Choosing the best material for kitchen appliances. Specific heat is a property that is specific to a given type of matter, and substances vary in their specific heat. We use a lowercase q to represent heat energy. Web q = s × m × δ t. Why should we care about the specific heat? The following formula shows how to calculate the heat necessary to increase an object's temperature by a certain change in temperature ( δt ). Connect the heating element up to the rest of the circuit as shown in the circuit diagram below, including an ammeter and voltmeter: Water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with each other and with other molecules. Web in other words, water has a high specific heat capacity, which is defined as the amount of heat needed to raise the temperature of one gram of a substance by one degree celsius. Choosing the best material for kitchen appliances. Some substances heat up quickly, while other substances heat up slowly. It describes how much heat must be added. The amount of heat needed to raise the temperature of 1 g water by 1 °c is has its own name, the calorie. The units for specific heat can either be joules per gram per degree \(\left( \text{j/g}^\text{o} \text{c} \right)\) or calories per gram per degree \(\left( \text{cal/g}^\text{o} \text{c} \right)\). The capability for a molecule to absorb heat energy is. How to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. Deduce which substance will have greatest temperature changed based on specific heat capacities. Q represents the amount of heat, s the specific heat ( ), m the mass of the substance in grams, and δ t the. The following formula shows how to calculate the heat necessary to increase an object's temperature by a certain change in temperature ( δt ). For conversion of units, use the specific heat online unit converter. Choosing the best material for kitchen appliances. The amount of heat absorbed or released by a substance depends directly on the type of substance, its. Web specific heat is the amount of heat energy necessary to raise one mass unit of a substance by one temperature unit. The units of specific heat capacity are j/(kg °c) or equivalently j/(kg k). Web q = s × m × δ t. Web defining specific heat, heat of fusion, and heat of vaporization. We use a lowercase q. Web the specific heat of a material is the amount of heat required to raise 1 kg of the material by 1°c. Web unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Web specific heat capacity represents how much heat energy is needed to raise the temperature of a substance by one degree celsius. Calculate unknown variables based on. The heat lost or gained by an object when its temperature changes by δt is: Q represents the amount of heat, s the specific heat ( ), m the mass of the substance in grams, and δ t the observed change in temperature. The symbol for specific heat is c. The specific heat measures how. It’s like a unique fingerprint for each material, revealing how well it “holds onto” or “releases” heat. Understand how body temperature can vary. It also describes their ability to store and release thermal energy. Distinguish the related properties of heat, thermal energy, and temperature. Define and distinguish specific heat and heat capacity, and describe the physical. Web in other words, water has a high specific heat capacity, which is defined as the amount of heat needed to raise the temperature of one gram of a substance by one degree celsius. The specific heat measures how much energy is stored in a heated object. Web specific heat, the quantity of heat required to raise the temperature of. Web the symbol for specific heat is \(c_p\), with the \(p\) subscript referring to the fact that specific heats are measured at constant pressure. Web q = s × m × δ t. The following formula shows how to calculate the heat necessary to increase an object's temperature by a certain change in temperature ( δt ). The amount of. Web the symbol for specific heat is \(c_p\), with the \(p\) subscript referring to the fact that specific heats are measured at constant pressure. Web the specific heat of a material is the amount of heat required to raise 1 kg of the material by 1°c. Different kinds of water, such as seawater, may have different specific heat. Web specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Web defining specific heat, heat of fusion, and heat of vaporization. The specific heat of water is 1 calorie (or 4.186 joules) per gram per celsius degree. For conversion of units, use the specific heat online unit converter. Distinguish the related properties of heat, thermal energy, and temperature. To relate heat transfer to temperature change. The heat lost or gained by an object when its temperature changes by δt is: The units for specific heat can either be joules per gram per degree \(\left( \text{j/g}^\text{o} \text{c} \right)\) or calories per gram per degree \(\left( \text{cal/g}^\text{o} \text{c} \right)\). The units of specific heat are usually calories or joules per gram per celsius degree. Web specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Web if a substance has a high specific heat capacity, it heats up and cools down slowly. It also describes their ability to store and release thermal energy. Web the most common variable for heat capacity is an uppercase c, and the most common units for it are j/°c, j/k, kj/°c, or kj/k.
Lesson Video Specific Heat Capacity Nagwa
PPT Molecules of Life PowerPoint Presentation, free download ID1321531

High Specific Heat (Water) — Properties & Examples Expii

Specific heat physics Britannica
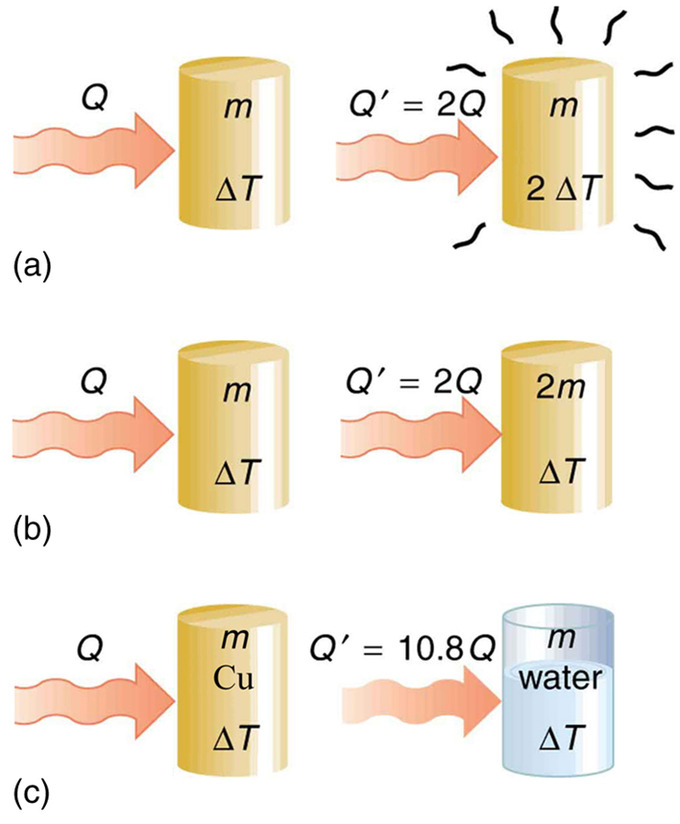
13.2 Specific Heat Physics LibreTexts
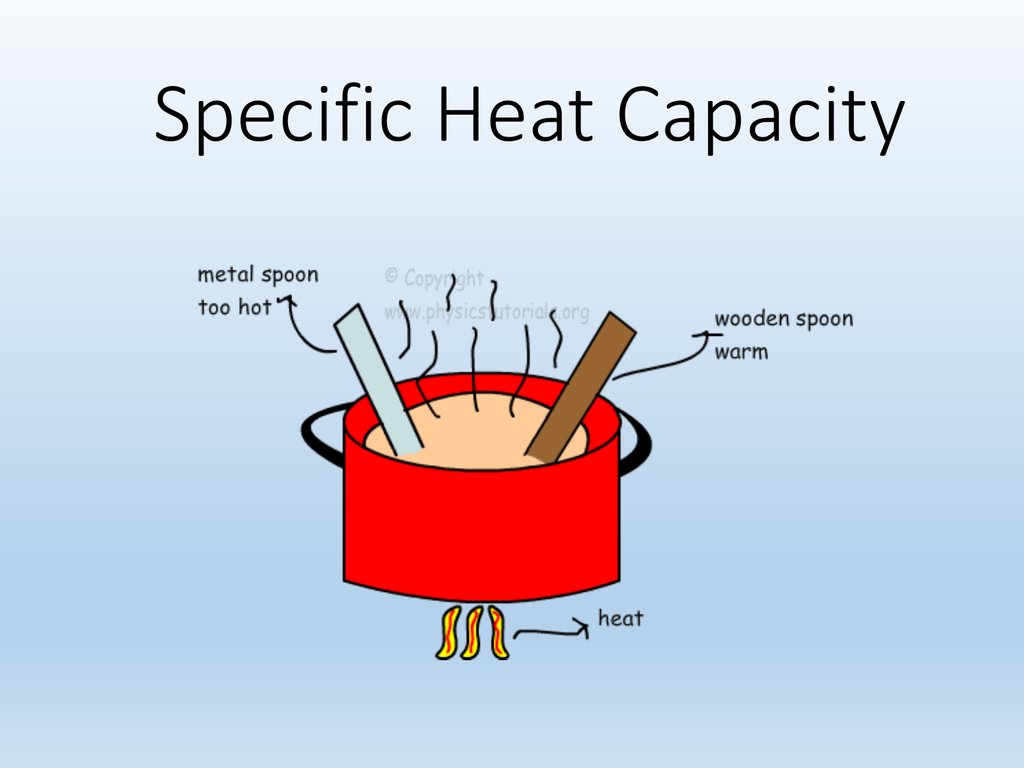
1.3 Specific Heat Capacity

42 examples of specific heat Blog Dicovery Education
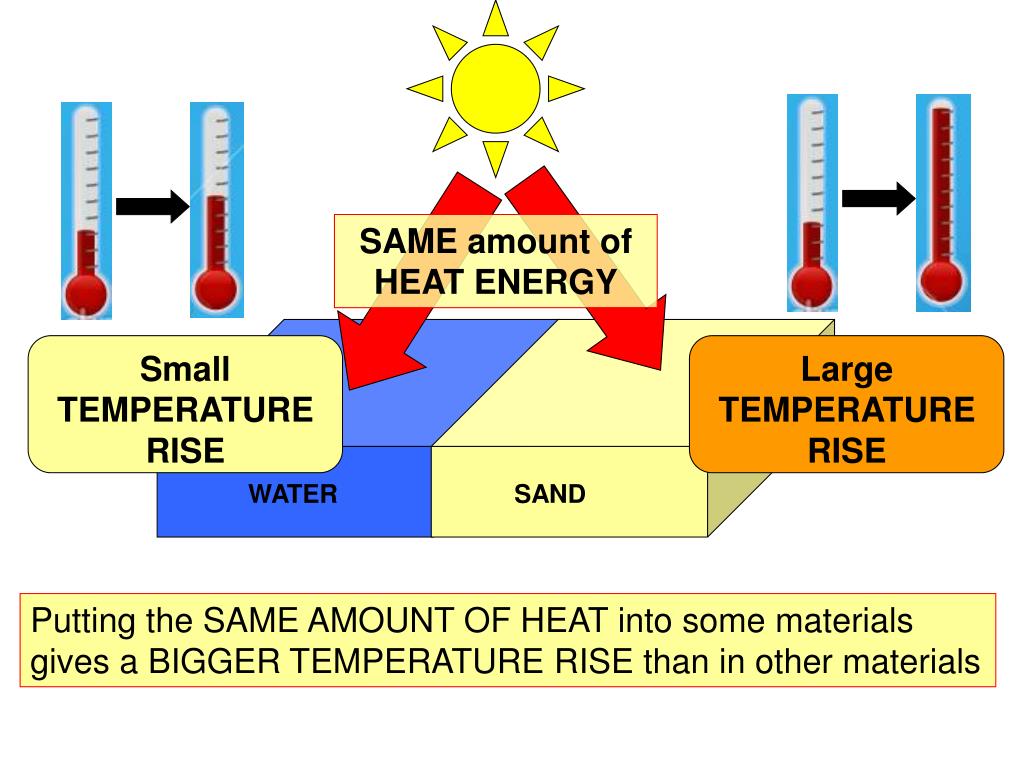
Specific Heat Capacity

Specific Heat Specific Heat At Constant Volume Specific Heat At
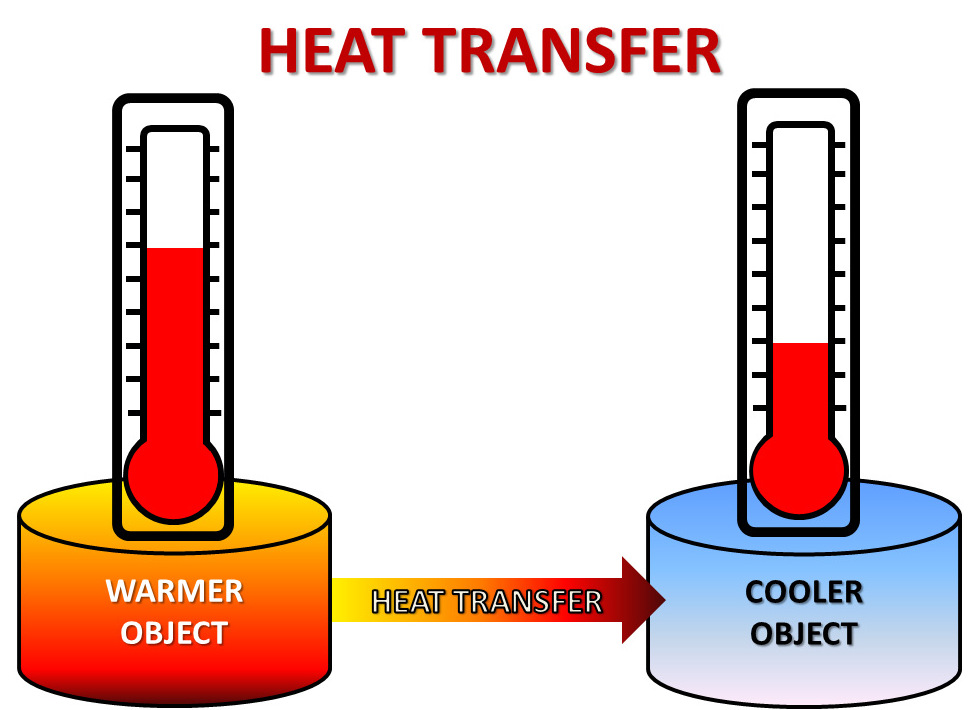
Lesson Plan of Heat and Temperature General Science Grade IV
Web Specific Heat Is Defined By The Amount Of Heat Needed To Raise The Temperature Of 1 Gram Of A Substance 1 Degree Celsius (°C).
It’s Like A Unique Fingerprint For Each Material, Revealing How Well It “Holds Onto” Or “Releases” Heat.
Web Q = S × M × Δ T.
This Is Why Water Is Valuable To Industries And In Your Car's Radiator As A.
Related Post: