How To Draw Covalent Bonds Lewis Structure
How To Draw Covalent Bonds Lewis Structure - Diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Steps for writing lewis structures. Add up all the valance electrons of the atoms involved. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Lewis dot structures can be produced by following a sequence of steps. The strength of a covalent bond depends on the overlap between the valence orbitals of the bonded atoms. Web using formal charges to distinguish viable lewis structures. Web here are the steps to draw a lewis structure. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Web using formal charges to distinguish viable lewis structures. Covalent bonds are formed when one electron from each atom forms an electron pair. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.the diagram is also called a lewis dot diagram, lewis dot formula, or. Determine the number of bonds in the molecule. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. When a chemical reaction occurs between two atoms, their valence electrons are reorganised so that a. Place one electron pair between each pair of adjacent atoms (as determined from the framework found in step 2) to form a single bond. Web yes, covalent bonds come in pairs which are represented by lines in lewis structures. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to. Web for anions add a number of electrons equal to the negative charge. •two types of bonds, ionicand covalent, and their formation can be used using lewis symbols. If i wanted to draw out a dot structure for c2h4, i would find carbon over here, and once again, carbon in is group four, so it has four valence electrons, so. If i wanted to draw out a dot structure for c2h4, i would find carbon over here, and once again, carbon in is group four, so it has four valence electrons, so i'm gonna go ahead and put in once carbon with four valence electrons, and. Number of electron pairs shared. Each h atom (group 1) has 1 valence electron,. Lewis dot structures can be produced by following a sequence of steps. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Web chemical bond formation. For cations subtract a number of electrons equal to the positive charge. Draw the lewis dot structure for the hydrogen atom. Determine the total number of valence electrons in the molecule or ion. How to draw the lewis dot structure for. A lewis structure can be drawn for any covalently bonded molecule, as well. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. Steps for writing lewis structures. Placing one bonding pair of electrons between the o atom and each h atom gives h:o:h, with 4 electrons left over. Web each h atom (group 1) has 1 valence electron, and the o atom (group 16). Web a bond is the sharing of 2 electrons. Single and multiple covalent bonds. Molecules can be represented using lewis structures, which show how electrons are arranged around the atoms in a molecule as bonded pairs of electrons (bonds) and lone pairs of electrons. The number of pairs of electrons shared between two atoms determines the type of the covalent. Web a bond is the sharing of 2 electrons. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Determine the total number of valence electrons in the molecule or ion. Steps for writing lewis structures. A lewis structure can be drawn for any covalently bonded molecule, as well. For example, two hydrogen atoms can. Draw the lewis dot structure for the hydrogen atom. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. How do we draw a covalent lewis dot structure? Determine the total number of valence electrons in the molecule or ion. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. Web in this video, we'll talk about multiple covalent bonds, and so we start the same way we did in the last video. Two (a pair of) valence electrons that are not used to form a covalent bond. Determine the total number of valence electrons in the molecule or ion. Single and multiple covalent bonds. Web solutions to example 10.4.1. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed. Since hydrogen is in group i it has one (1) valence electron in. How to draw the lewis dot structure for. Web lecture b1 systematic method for creating lewis dot structures. The sulfur has 2 lone pairs while the chlorines have 3 lone pairs each.The Covalent Bond CK12 Foundation

Lewis Structure For Seh Cf S Lewis Structure How To Draw The Lewis My

9.5 Covalent Bonding Lewis Structures YouTube

How to Draw a Lewis Structure
![[DIAGRAM] Drawing Lewis Dot Diagrams For Covalent Bonds](https://image1.slideserve.com/1587654/lewis-dot-diagrams-covalent-bonds-l.jpg)
[DIAGRAM] Drawing Lewis Dot Diagrams For Covalent Bonds

Lewis Diagrams of Covalent Compounds YouTube
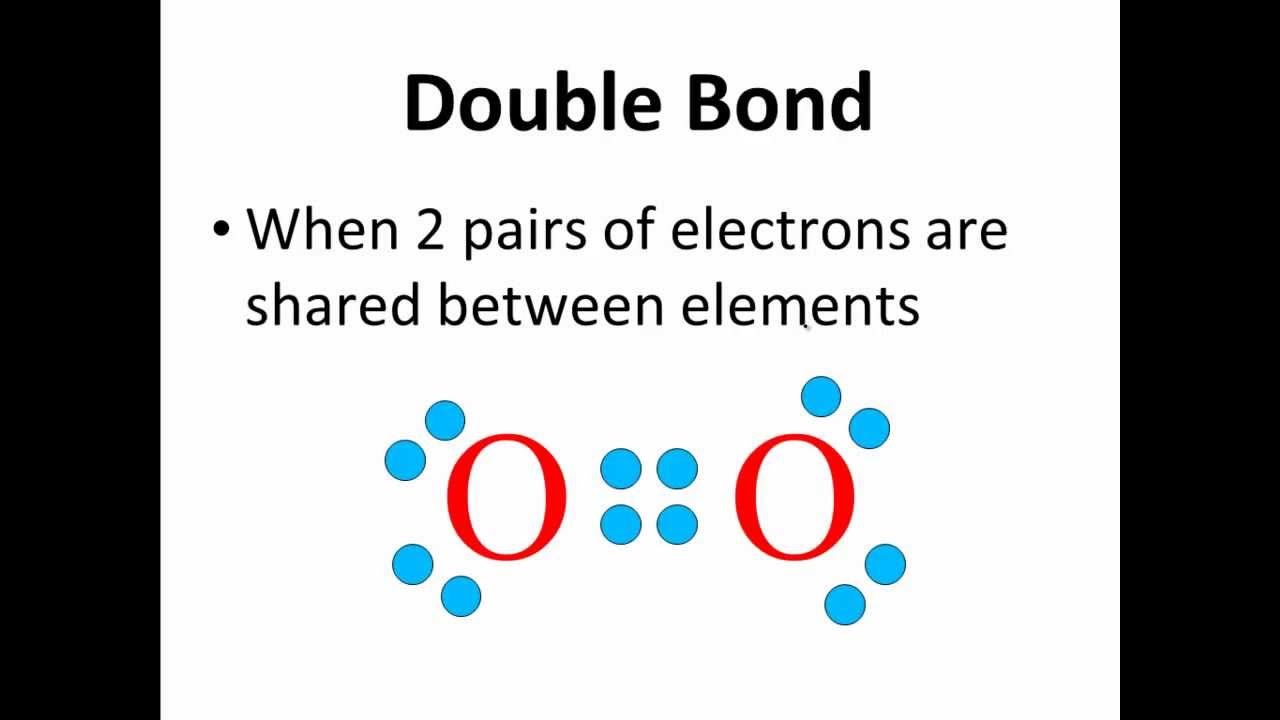
Lewis Dot Structures for Covalent Compounds Part 1 CLEAR & SIMPLE

Covalent Bonds In Electron Dot Structures (Lewis
The Covalent Bond CK12 Foundation

How to Draw Lewis Dot Structure of Covalent Compounds Chemical
Two (A Pair Of) Valence Electrons That Are Not Used To Form A Covalent Bond.
Let’s Produce A Lewis Dot Structure For:
Lewis Dot Structures Can Be Produced By Following A Sequence Of Steps.
Web Yes, Covalent Bonds Come In Pairs Which Are Represented By Lines In Lewis Structures.
Related Post:

