How To Draw Hybridization Orbitals
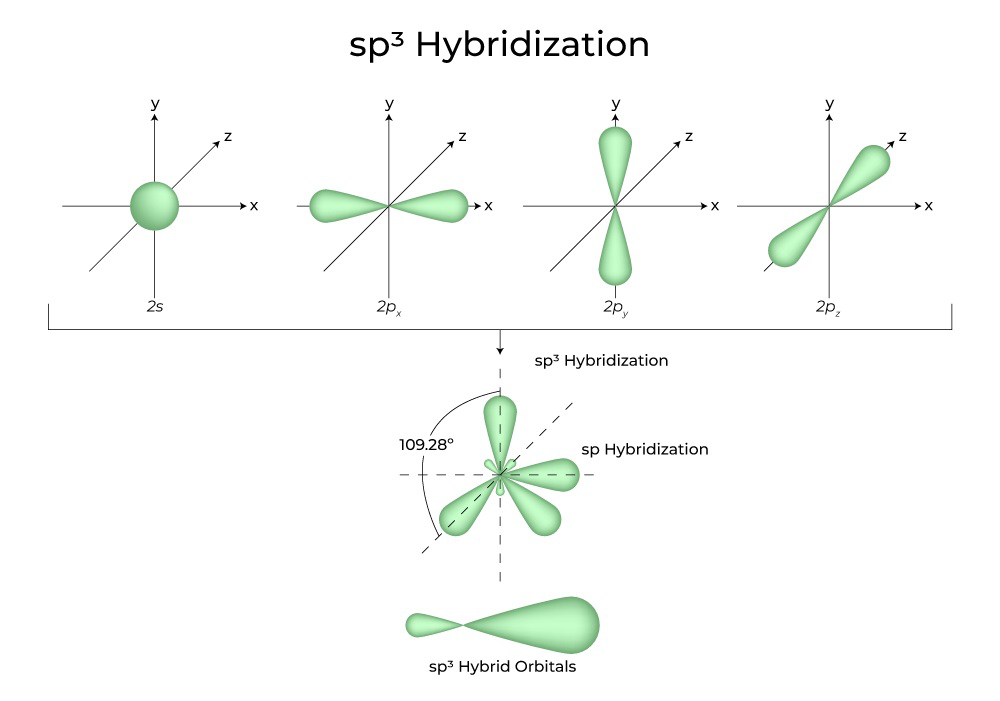
How To Draw Hybridization Orbitals - In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Unhybridized orbitals overlap to form π bonds. This makes it a challenge to draw, but i will show you the strategies in the video.in particular, you need to sho. In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Draw orbital box diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. An sp3 hybrid orbital is composed of four atomic orbitals, one s and three p, so the s character is ¼ or 25% (making the p character ¾ or 75%). Draw the valence electron configuration for the ground state: Taking the sum and difference of an ns and an np atomic orbital where n = 2 gives two equivalent sp hybrid orbitals oriented at 180° to each other. Sp 2 hybridization can explain the trigonal planar structure of molecules. In beh 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. Hybrid orbitals overlap to form σ bonds. This makes it a challenge to draw, but i will show you the strategies in the video.in particular, you need to sho. Web the carbon atoms of c2h2 are sp hybridized. Sp 2 hybridization can explain the trigonal planar structure of molecules. Draw orbital box diagrams showing how combinations of an atomic s. Web steps to draw hybridized orbitals: Web sp 2 hybridization. In it, the 2s orbitals and two of the 2p orbitals hybridize to form three sp orbitals, each consisting of 67% p and 33% s character. From the valence electron configuration of the central atom and the number of electron pairs, determine the hybridization. The nucleus resides just inside the. Note that each sp orbital contains one lobe that is significantly larger than the other. Sp 2 hybridization can explain the trigonal planar structure of molecules. Web steps to draw hybridized orbitals: The frontal lobes align themselves in the trigonal planar structure, pointing to the corners of a triangle in order to minimize. Sp 2 hybridization can explain the trigonal. This makes it a challenge to draw, but i will show you the strategies in the video.in particular, you need to sho. Web the nitrogen atom of ammonia (nh3) is sp3 hybridized. Resonance structures, on the other hand, are different ways of. It explains how to find the hybridi. Unhybridized orbitals overlap to form π bonds. An sp3 hybrid orbital is composed of four atomic orbitals, one s and three p, so the s character is ¼ or 25% (making the p character ¾ or 75%). Web directory of chem help asap videos: Sp 2 hybridization can explain the trigonal planar structure of molecules. It explains how to find the hybridi. In beh 2, we can. There are two oxygen atoms bonded to the center carbon atom and there are zero lone pairs around the carbon center. Sp 2 hybridization can explain the trigonal planar structure of molecules. Note that each sp orbital contains one lobe that is significantly larger than the other. In sp² hybridization, one s orbital and two p orbitals hybridize to form. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Sp 2 hybridization can explain the trigonal planar structure of molecules. The frontal lobes align themselves in the trigonal planar structure, pointing to the corners of a triangle in order. In beh. Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. There are two oxygen atoms bonded to the center carbon atom and there are zero lone pairs around the carbon center. In this video, we use both of these methods to determine the. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Web directory of chem help asap videos: Web this organic chemistry video tutorial explains the hybridization of atomic orbitals. Each hybrid orbital is oriented primarily in just one direction. Web the nitrogen. The set of two sp orbitals are oriented at 180°, which is consistent with the. Web figure 11.3.1 11.3. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Web hybridization of s and p orbitals. Figure 8.11 this alternate way of. There are two oxygen atoms bonded to the center carbon atom and there are zero lone pairs around the carbon center. Web the carbon atoms of c2h2 are sp hybridized. Determine the steric number and hybridization of the center atom. Sp 2 hybridization can explain the trigonal planar structure of molecules. It discusses how to determine the number of sigma and pi bonds in a mol. Web each individual hybrid orbital is a combination of multiple atomic orbitals and has different s and p character affecting their shape, length, and acidic properties. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Web steps to draw hybridized orbitals: Web this organic chemistry video tutorial explains the hybridization of atomic orbitals. Hybrid orbitals overlap to form σ bonds. Note that each sp orbital contains one lobe that is significantly larger than the other. Taking the sum and difference of an ns and an np atomic orbital where n = 2 gives two equivalent sp hybrid orbitals oriented at 180° to each other. Web this video explains how to decide what the hybridization around an atom will be and also discusses the bonding picture of ethylene (ethene). Sp 2 hybridization can explain the trigonal planar structure of molecules. In it, the 2s orbitals and two of the 2p orbitals hybridize to form three sp orbitals, each consisting of 67% p and 33% s character. In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character.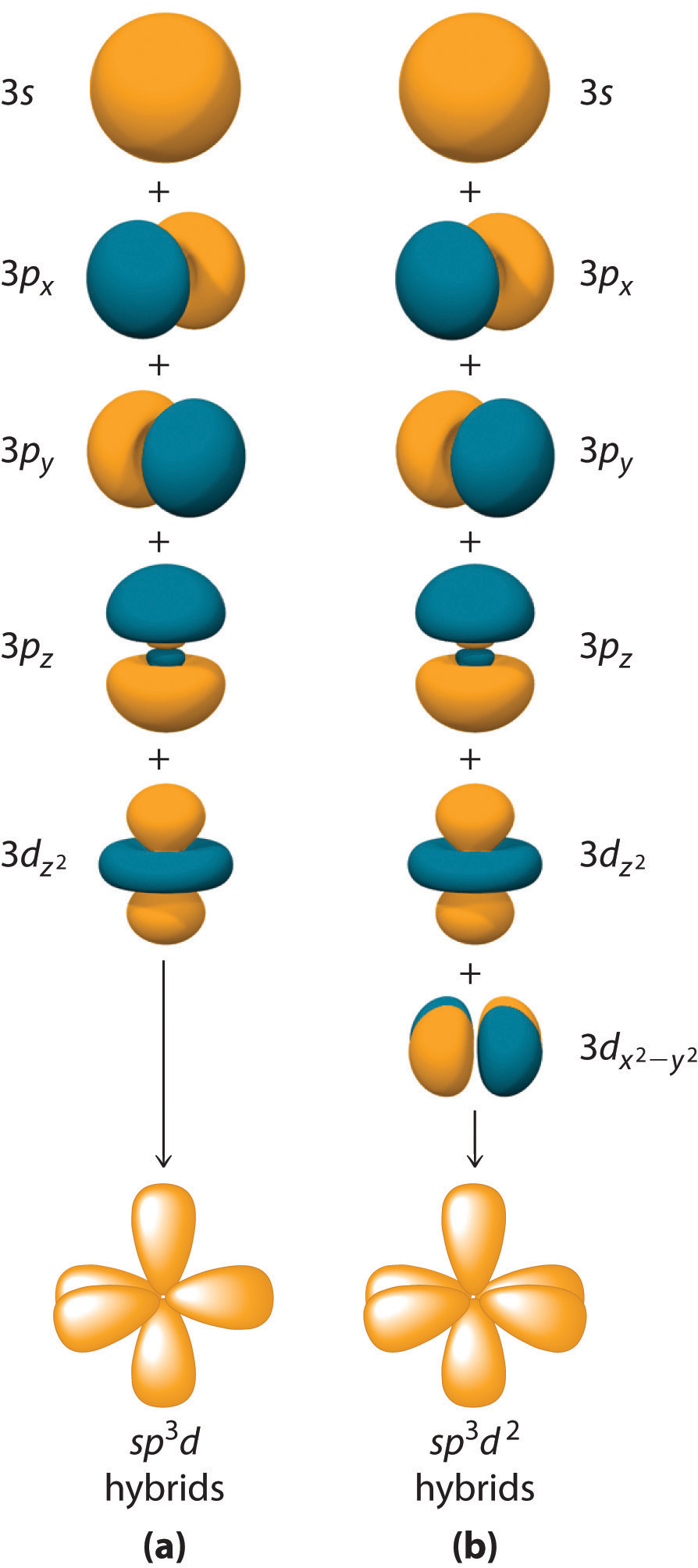
Localized Bonding and Hybrid Atomic Orbitals
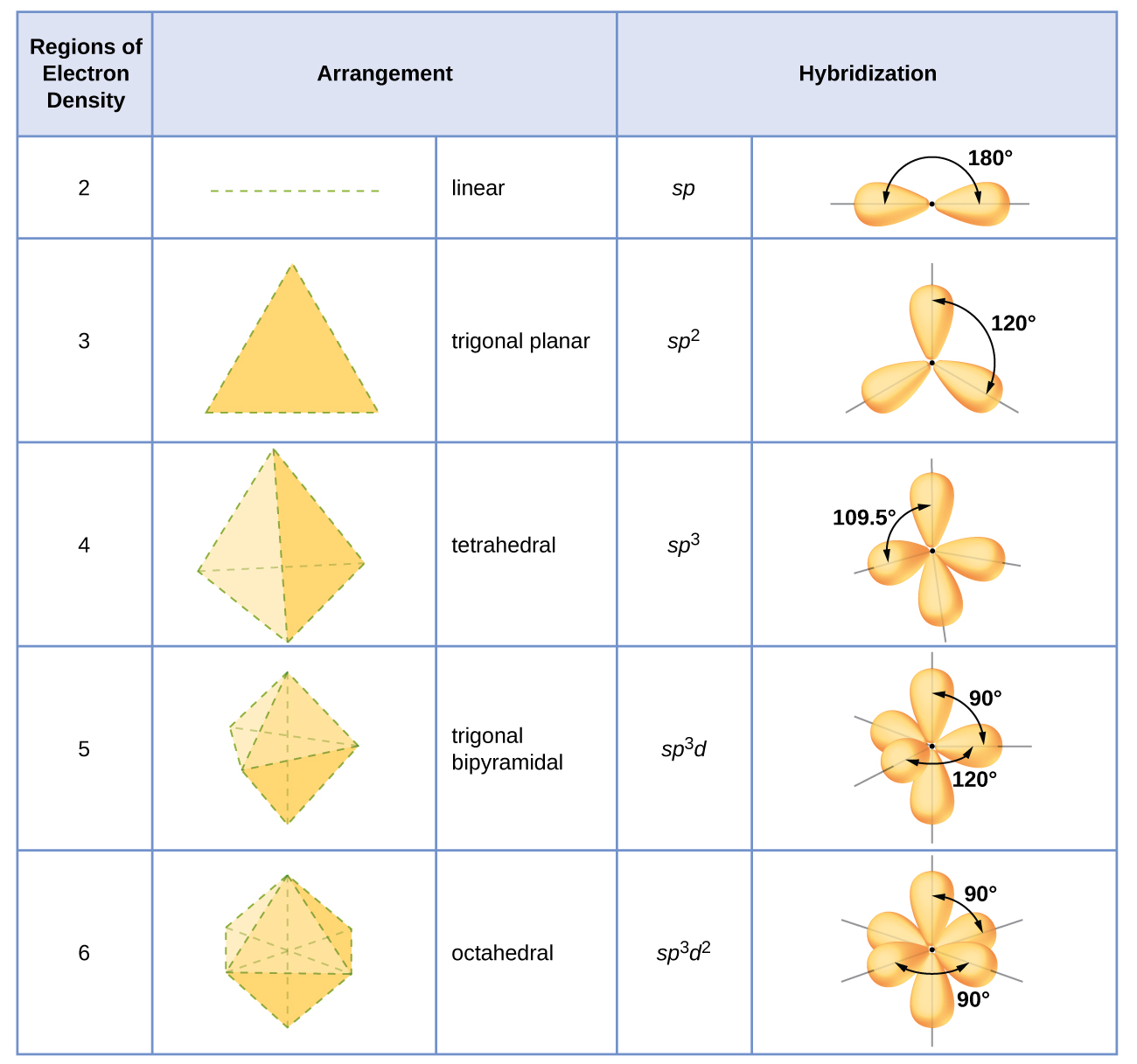
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
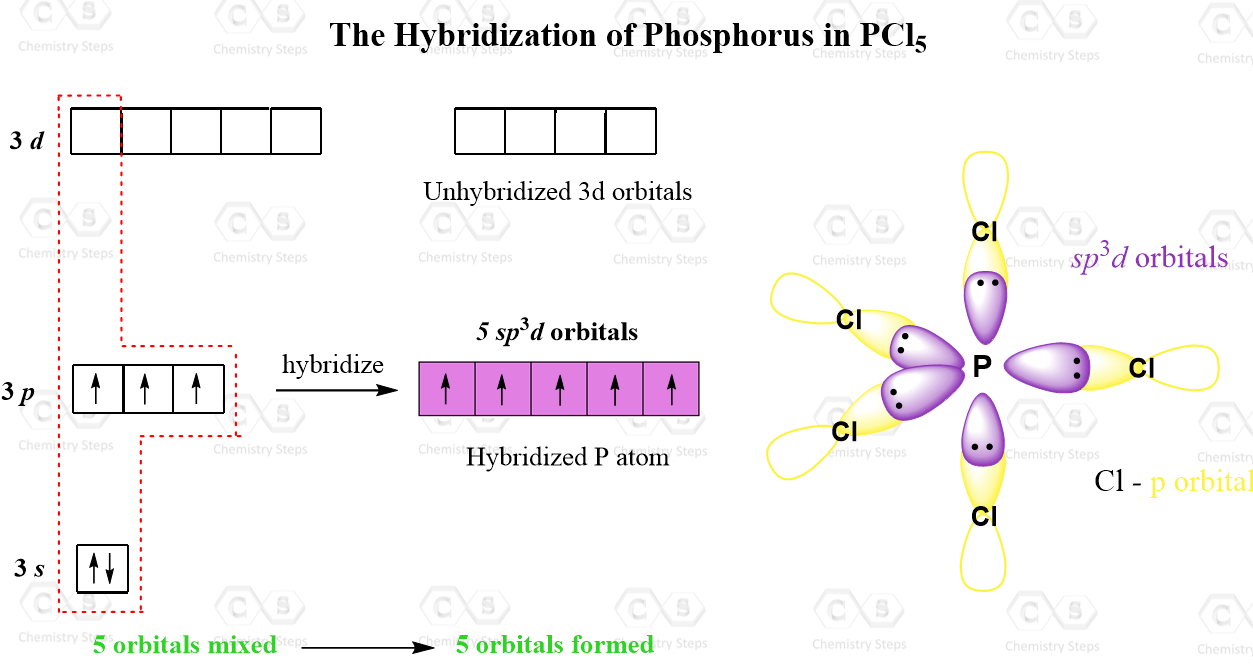
Hybridization of Atomic Orbitals Chemistry Steps
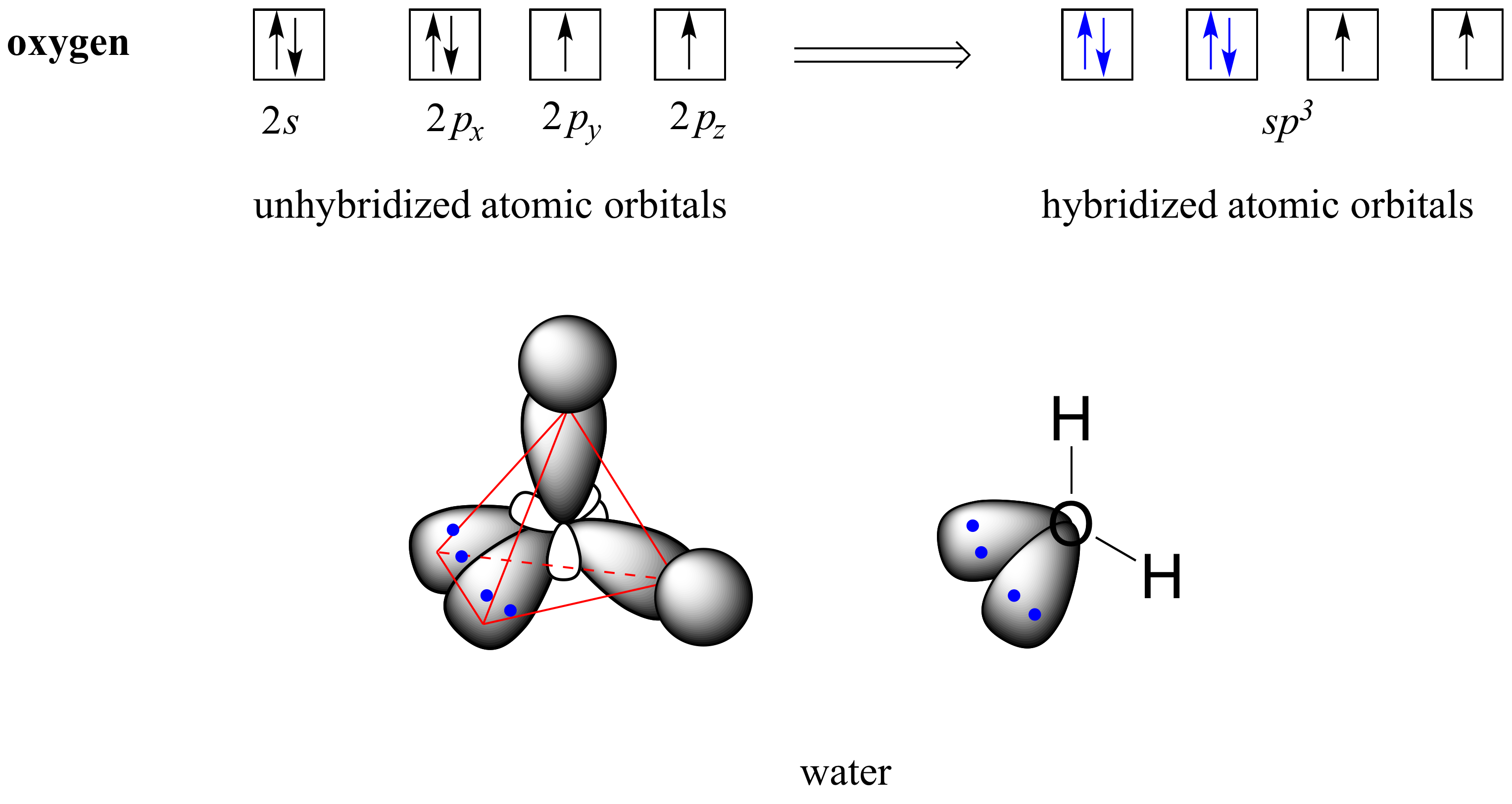
2.2 Hybrid orbitals Chemistry LibreTexts
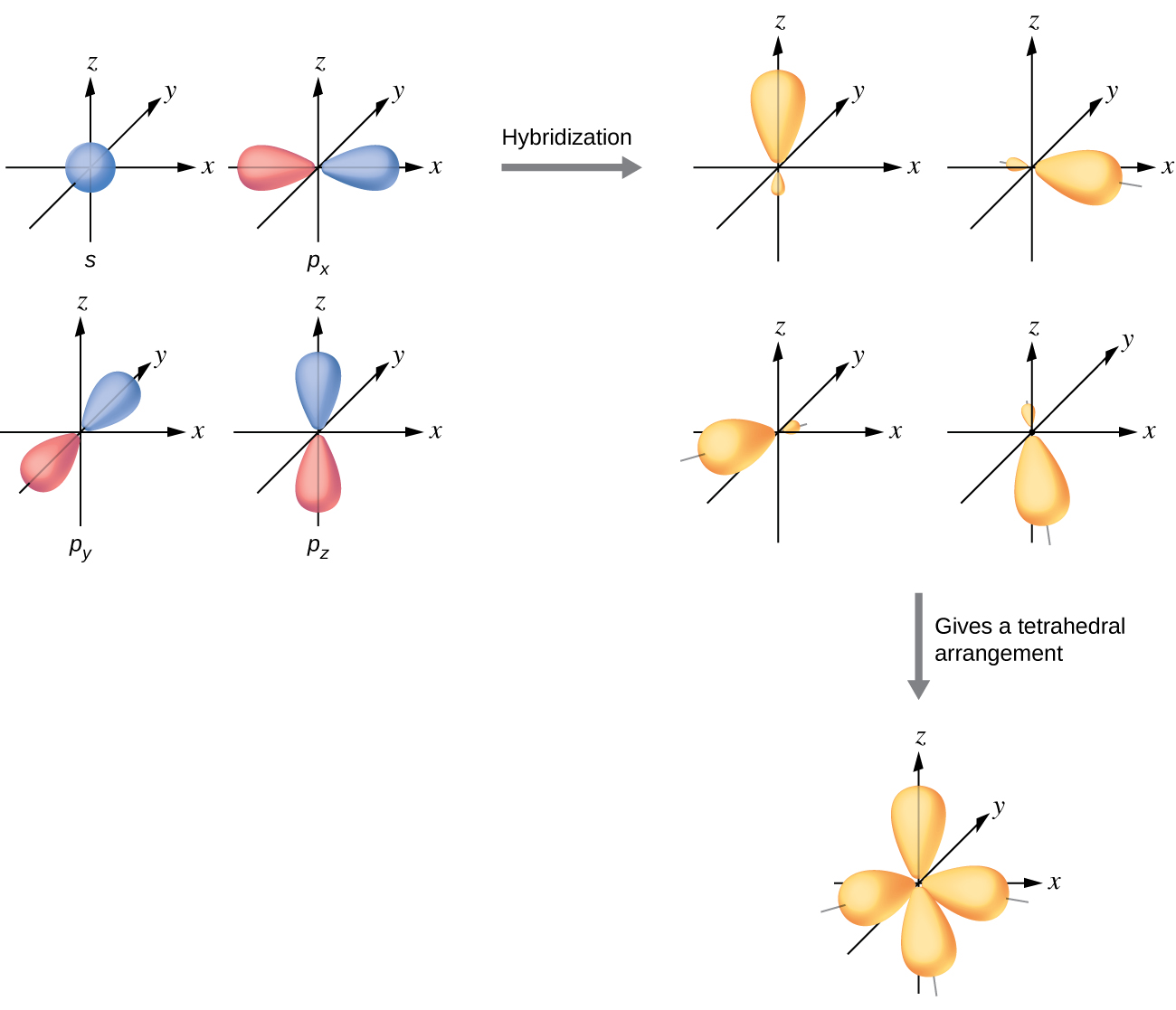
8.2 Hybrid Atomic Orbitals Chemistry

Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp Sp2 Sp3 YouTube
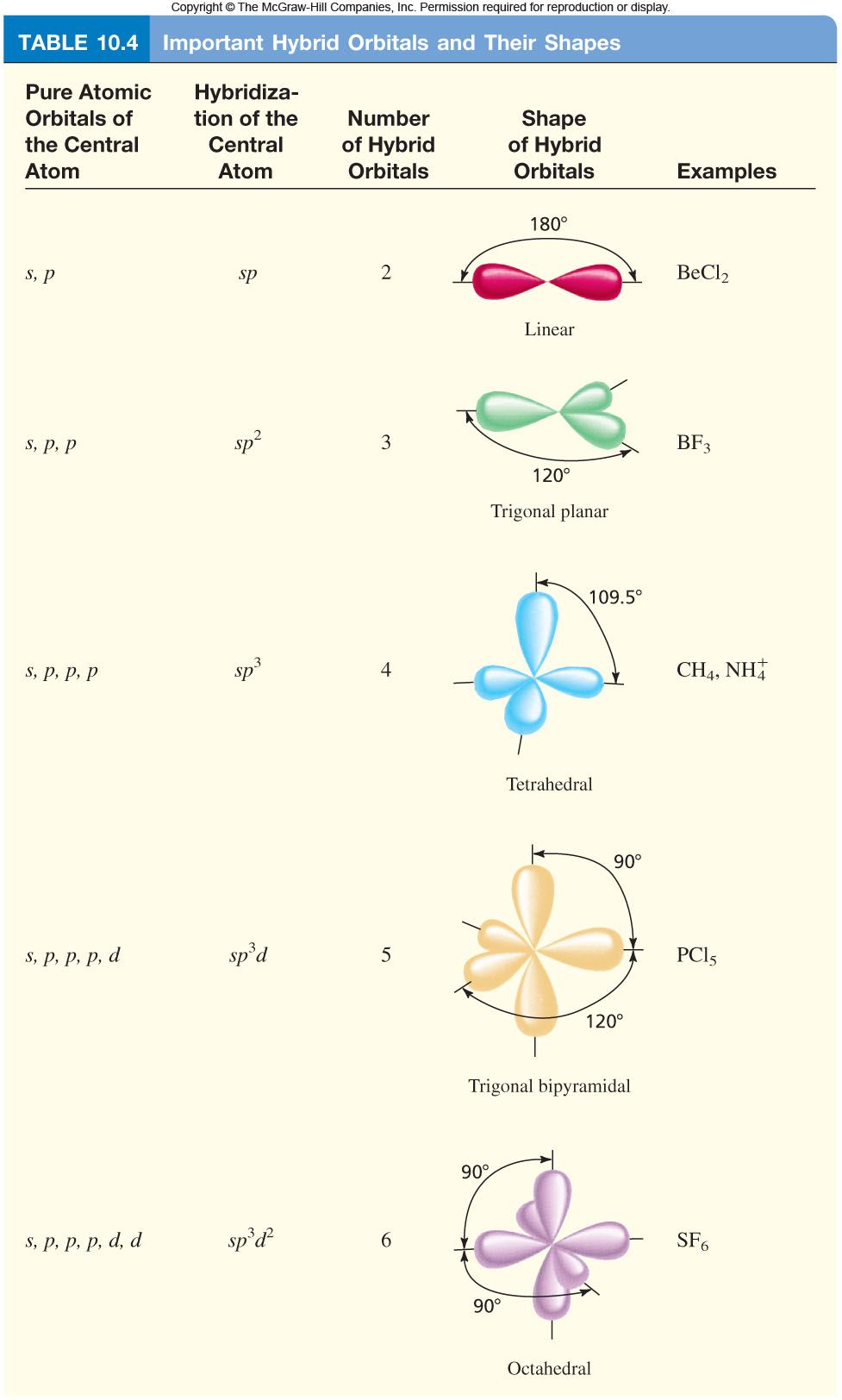
Orbital Hybridization "Cheat Sheet"? + Example
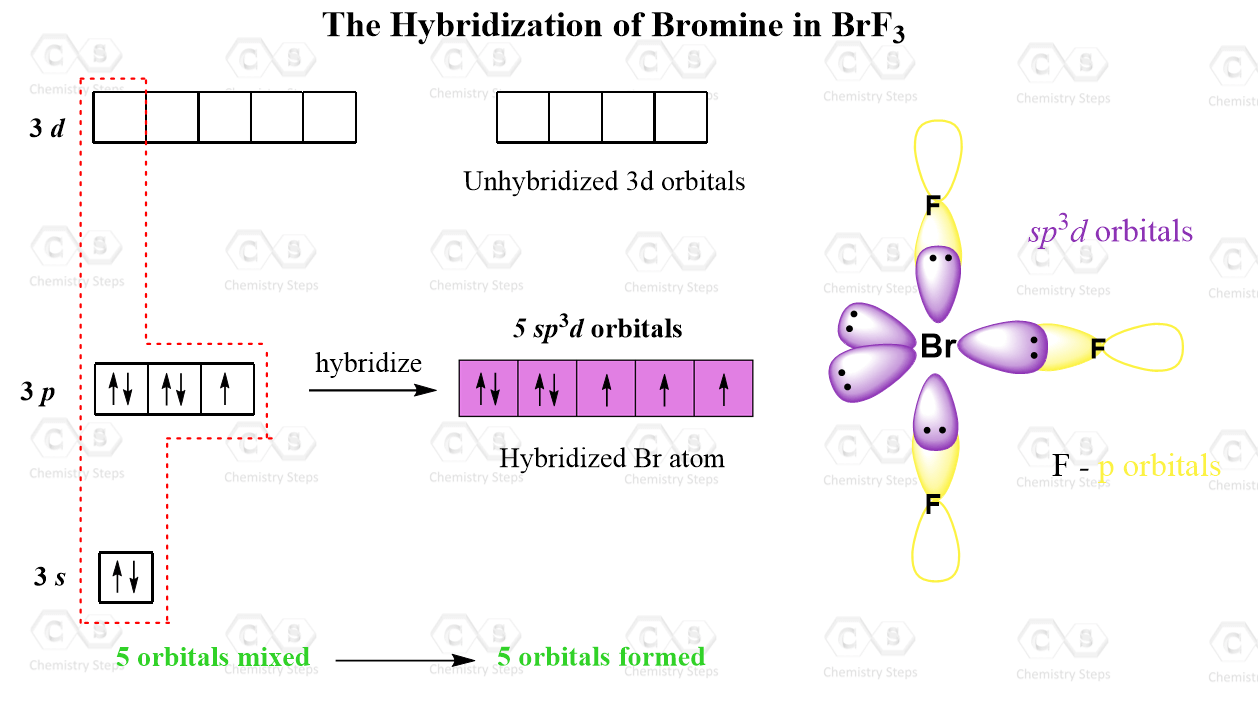
Hybridization of Atomic Orbitals Chemistry Steps
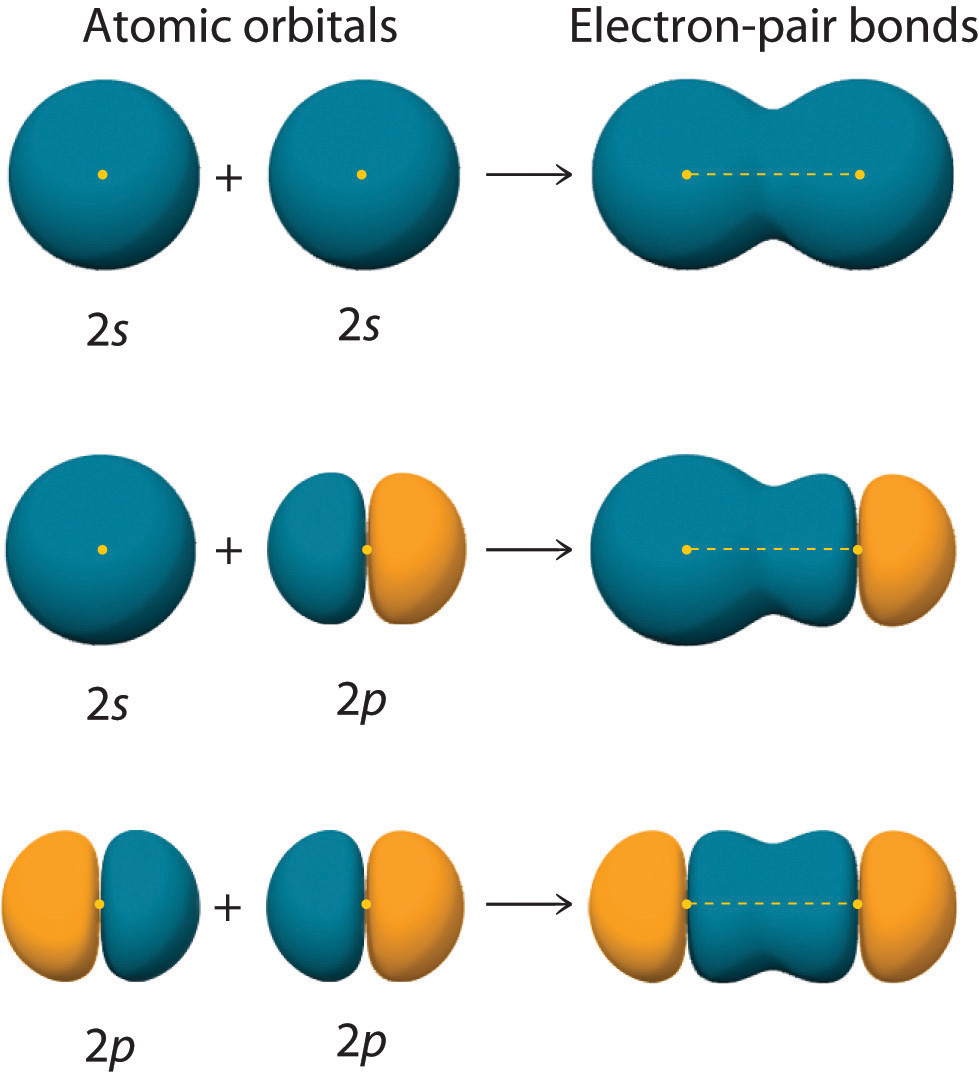
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
Sp Orbitals
Merge The Orbitals Depending On The Type Of Hybridization:
This Type Of Hybridization Is Required Whenever An Atom Is Surrounded By Three Groups Of Electrons.
An Sp3 Hybrid Orbital Is Composed Of Four Atomic Orbitals, One S And Three P, So The S Character Is ¼ Or 25% (Making The P Character ¾ Or 75%).
The Nucleus Resides Just Inside The Minor Lobe Of.
Related Post: