How To Draw Lewis Structures For Ions
How To Draw Lewis Structures For Ions - Web this chemistry video explains how to draw the lewis structures of ionic compounds. Lewis dot structures for polyatomic ions and molecules : Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis dot structure for the hydrogen atom. @ incorrect your structure is missing one or more atoms. Draw the lewis structure for. Divide the number of bonding electrons by 2 to get the number of bonds: Draw the resonance structures and use curly arrows to track the movement of electrons between structures. Web rules for drawing lewis structures. Web here are the steps to draw a lewis structure. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. However, the rules for doing this are a little different. In this section, we will. The tendency to form species that have. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Lewis dot structures for polyatomic ions and molecules : Web a lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms. When constructing a lewis diagram, keep. We start by calculating the total number of valence electrons using the periodic table. Lewis structures of polyatomic ions: It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. The electrons denoted as dots are called lone. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Web draw lewis structures for the following ions. Draw the lewis dot structure for the hydrogen atom. When drawing polyatomic ions, we do pretty much the same thing as we did with neutral covalent compounds. The example is for the nitrate ion. Web the strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Write the full electron configurations of the following substances nitrogen atom: Web draw lewis structures for the following ions.. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. Draw the resonance structures and use curly arrows to track the movement of electrons between structures. Web here are the steps to draw a lewis structure. First, the total number of valence electrons present in the molecule is calculated by adding the. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Write the full electron configurations of the following substances nitrogen atom: Web rules for drawing lewis structures. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central. Web two chlorines are bonded to a sulfur. Polyatomic ions are ions that contain more than one atom. Polyatomic ions are molecular ions or coval. Web these structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. Web the strength of ionic bonding depends on the magnitude. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web lewis dot structure of sulfide ion. Resonance structures are possible for the ions in q 2. Web the strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. You can check your work on each atom. Count each bond as 2 electrons (double and triple bonds as 4 and 6 electrons, respectively). (note that we denote ions with brackets around the structure, indicating the charge outside the brackets:) when several arrangements of atoms are possible, as for cho 2. There are 2 steps to solve this one. However, the rules for doing this are a little. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. One way to do this is to write the lewis symbols for all of the atoms in the formula, and count up all the dots. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web two chlorines are bonded to a sulfur. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share. When drawing polyatomic ions, we do pretty much the same thing as we did with neutral covalent compounds. @ incorrect your structure is missing one or more atoms. You can check your work on each atom by counting each dot as 1 electron and each bond as 2 electrons. In this section, we will. Electrons denoted as lines are bonds and show the sharing of two electrons between. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. First of all, a correct count of all valence electrons is essential. Web these structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. Lewis structures of polyatomic ions: Resonance structures are possible for the ions in q 2.
3 Ways to Draw Lewis Dot Structures wikiHow

OH Lewis Structure How to Draw the Lewis Dot Structure for the

Draw The Lewis Structure For The Following Molecules And Ions And

ClO2 Lewis Structure How to Draw the Lewis Structure for ClO2

3 Ways to Draw Lewis Dot Structures wikiHow

How to Draw a Lewis Structure
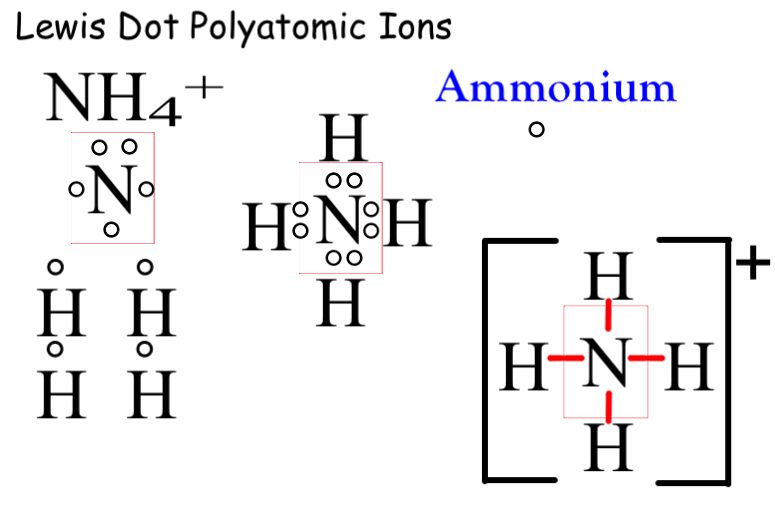
How do you draw lewis structures for polyatomic ions? Socratic

Lewis Dot Structures of Atoms and Ions YouTube
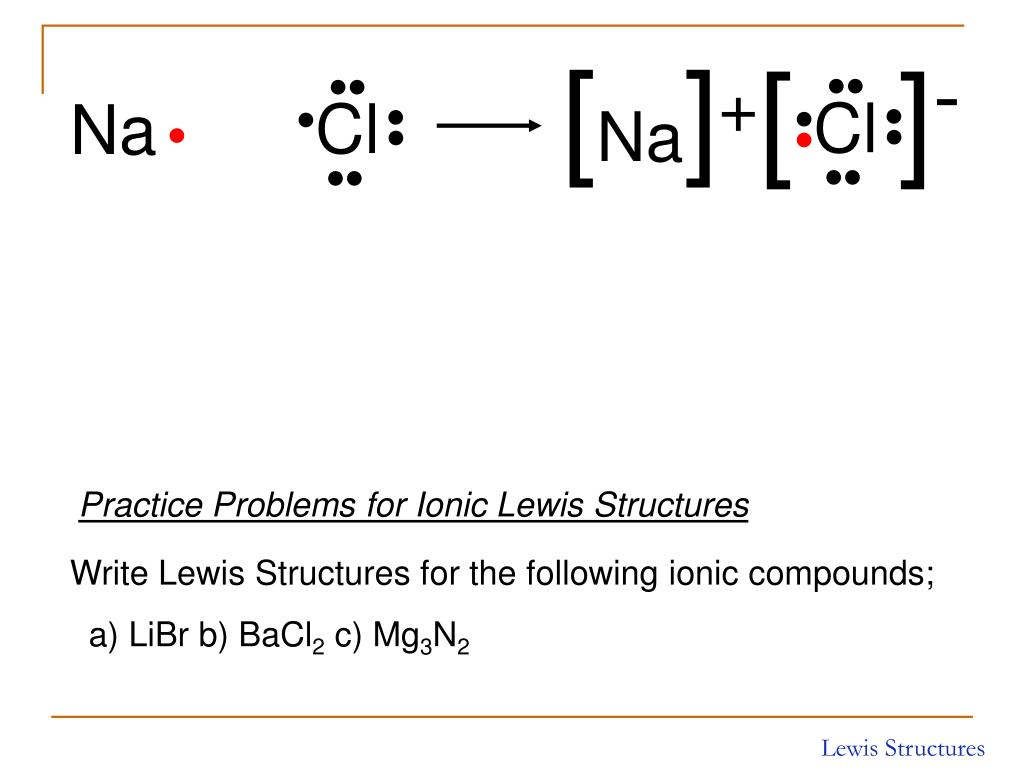
How To Draw Lewis Structures For Ionic Compounds slideshare
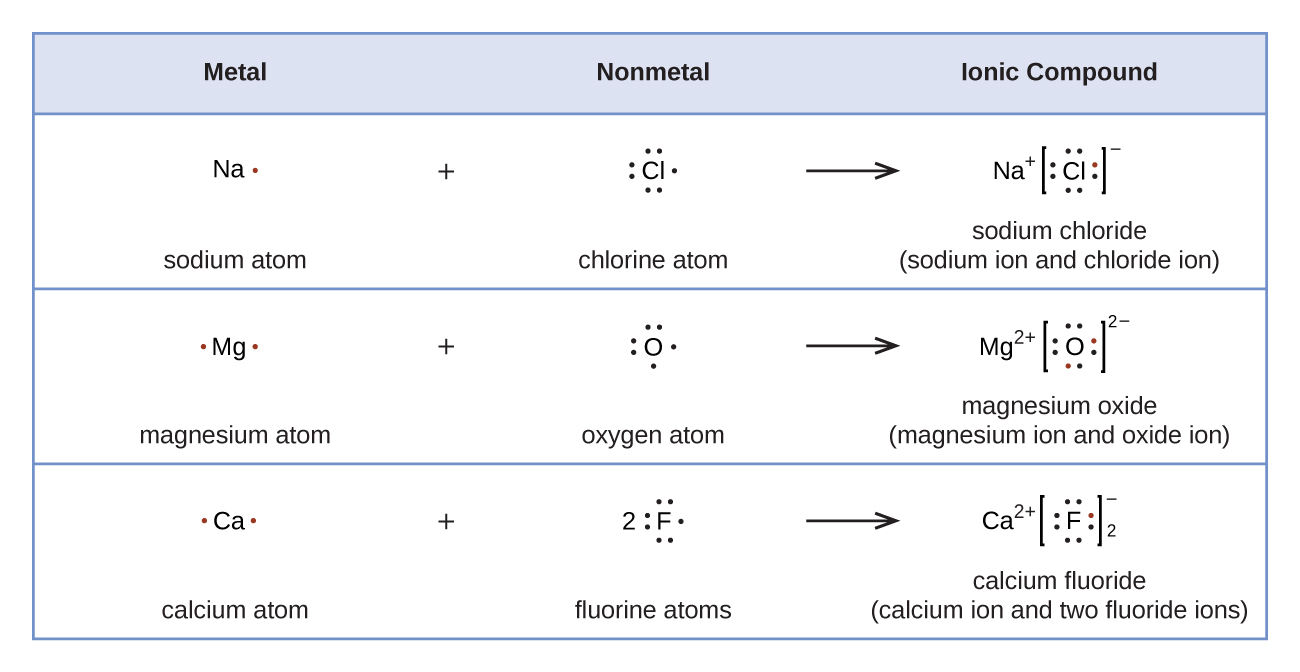
10.1 Lewis Structures and the Octet Rule Chemistry LibreTexts
Web Lewis Dot Structure Of Sulfide Ion.
Web How To Draw Lewis Structures.
Given A Chemical Formula Corresponding To A Molecule Or Molecular Ion, Draw A Lewis Structure.
First, The Total Number Of Valence Electrons Present In The Molecule Is Calculated By Adding The Individual Valencies Of.
Related Post: