Sodium Atom Drawing
Sodium Atom Drawing - Electrons are the negatively charged particles that orbit the nucleus of an atom. Atomic number of any element refers to the number of electrons in an atom of that element they are having. The element sodium is in the alkali metal group of the periodic table. 31k views 3 years ago. Molview consists of two main parts, a structural formula editor and a 3d model viewer. (or) the identical radii of zirconium (zr) and hafnium (hf). Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. The atomic radii of 3rd row transition series elements are almost similar to that of 2nd row transition series elements. Atoms (basic chemistry tutorial 1) focused on the general structure of atoms. If you’ve already watched the video, click here, or scroll down below the video to start interacting. The number of protons is always equal to. By going through the periodic table, we see that the lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. 11), the most common isotope of the element. Similarly, neon has a complete outer 2n shell containing eight electrons. First, let us determine the number of protons in the sodium atom. 61k views 4 years ago. In this video we'll look at the atomic. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Electrons are the negatively charged particles that orbit the nucleus of an atom. 11), the most common isotope of the element sodium. The name is derived from the english word 'soda'. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. The structural formula editor is surround by three toolbars. Find the number of protons, electrons, and neutrons in the sodium atom. Web steps to draw the bohr model of sodium atom. 11), the most common isotope of the element sodium. Memorization over the winter break. In this video we'll look at the atomic. Web steps to draw the bohr model of sodium atom. 11), the most common isotope of the element sodium. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The first step, however, is to teach them how to draw basic models of atoms. In this video, we determine how to. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. 11 electrons (green) bind to the. The atomic radii of 3rd row transition series elements are almost similar to that of 2nd row transition series elements. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. The atomic radii of 3rd row transition series elements are almost similar to that of 2nd row transition series elements. Web to write the orbital diagram for the sodium atom (na) first we need to write the electron configuration for. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. For the na+ structure use the periodic table to find the total number of valence electrons for. Web 32k views 9 years ago. Members of a group typically have similar properties and electron configurations in their outer shell. Once you’ve drawn. The nucleus consists of 11 protons (red) and 12 neutrons (blue). In this video, we determine how to draw the orbital diagram of sodium. The first step, however, is to teach them how to draw basic models of atoms. The sodium atom (na) is commonly used for examples and practice problems in chemistry. Then play a game to test your. Always write the atomic number of the element first. The name is derived from the english word 'soda'. Web to draw the bohr model of sodium we must first find out the number of all the atomic particles present in the sodium atom. First, let us determine the number of protons in the sodium atom. 11), the most common isotope of the element sodium. Web 140k views 5 years ago. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. I show you where sodium is on the periodic table and how to determine how many valence electrons it has. 1.8k views 2 years ago. In this video we'll look at the atomic. Draw the bohr diagram of an atom with 18 electrons or fewer. Web so, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. The first step, however, is to teach them how to draw basic models of atoms. The atomic radii of 3rd row transition series elements are almost similar to that of 2nd row transition series elements. In contrast, chlorine and sodium have seven and one electrons in their outer shells, respectively. Here, you’ll learn how to determine an atom’s specific structure, which will set us up for learning about chemical bonds in the next tutorials.
Atomic Structure Of Sodium Ion
:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)
Atom Diagrams Electron Configurations of the Elements
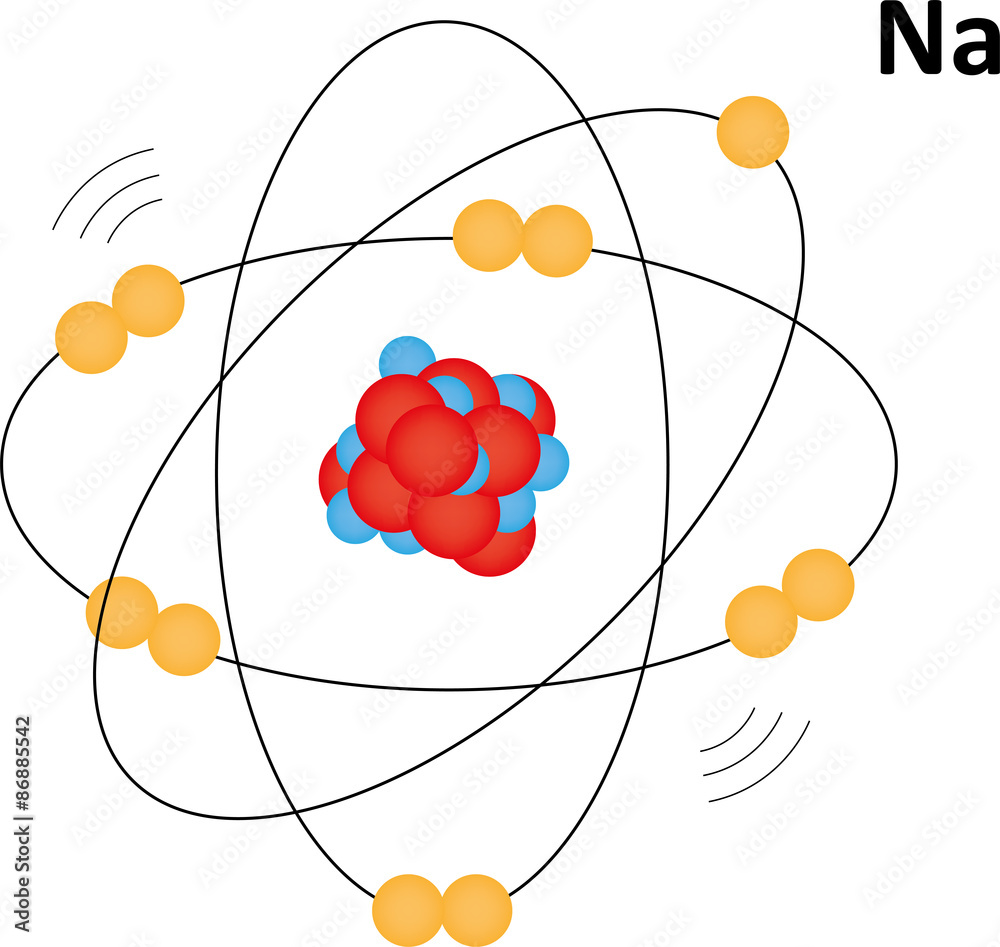
Sodium Atom Stock Illustration Adobe Stock
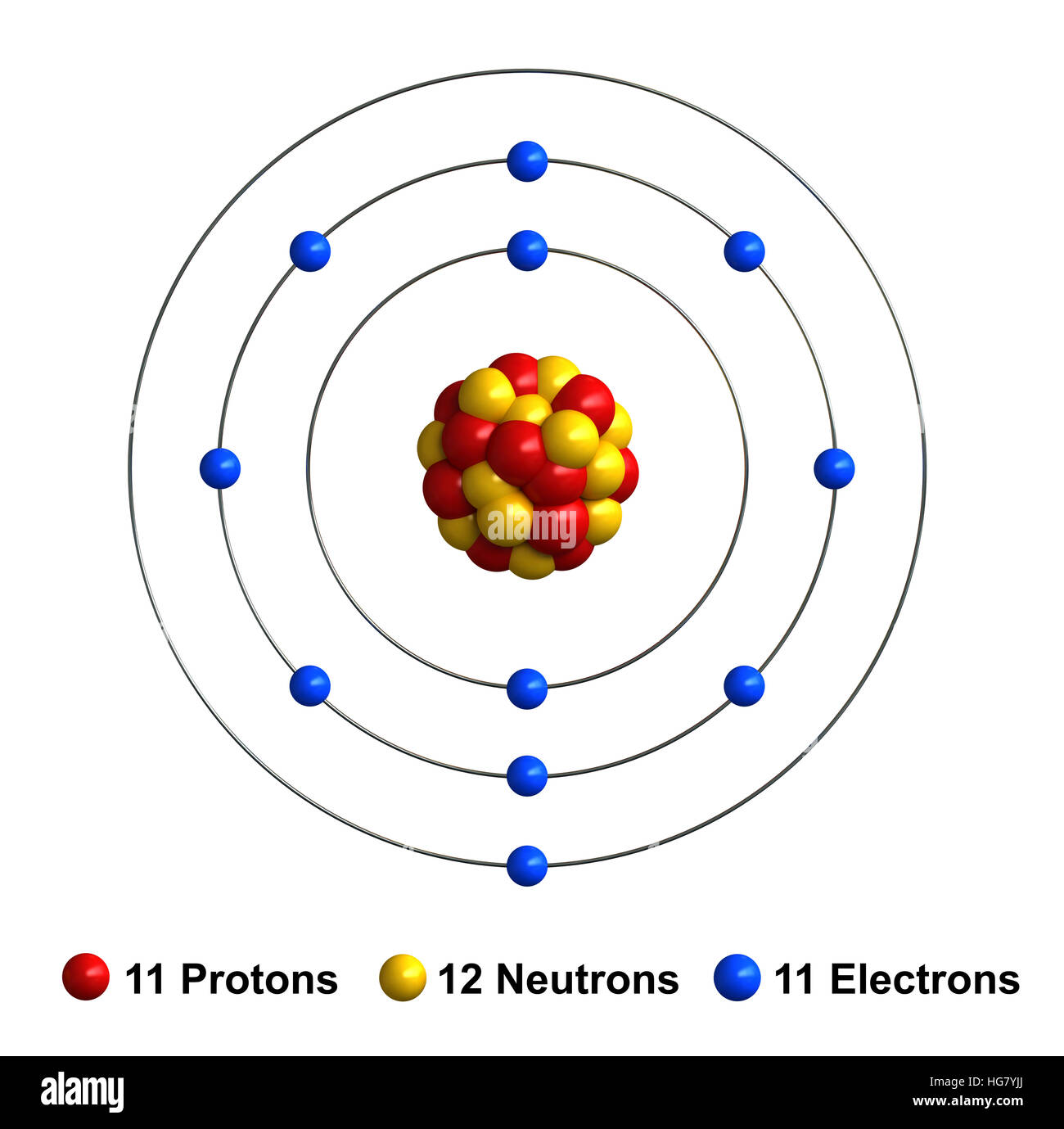
3d render of atom structure of sodium isolated over white background

A simple representation of the sodium atom. Download Scientific Diagram

sodium electron configuration Newton Desk
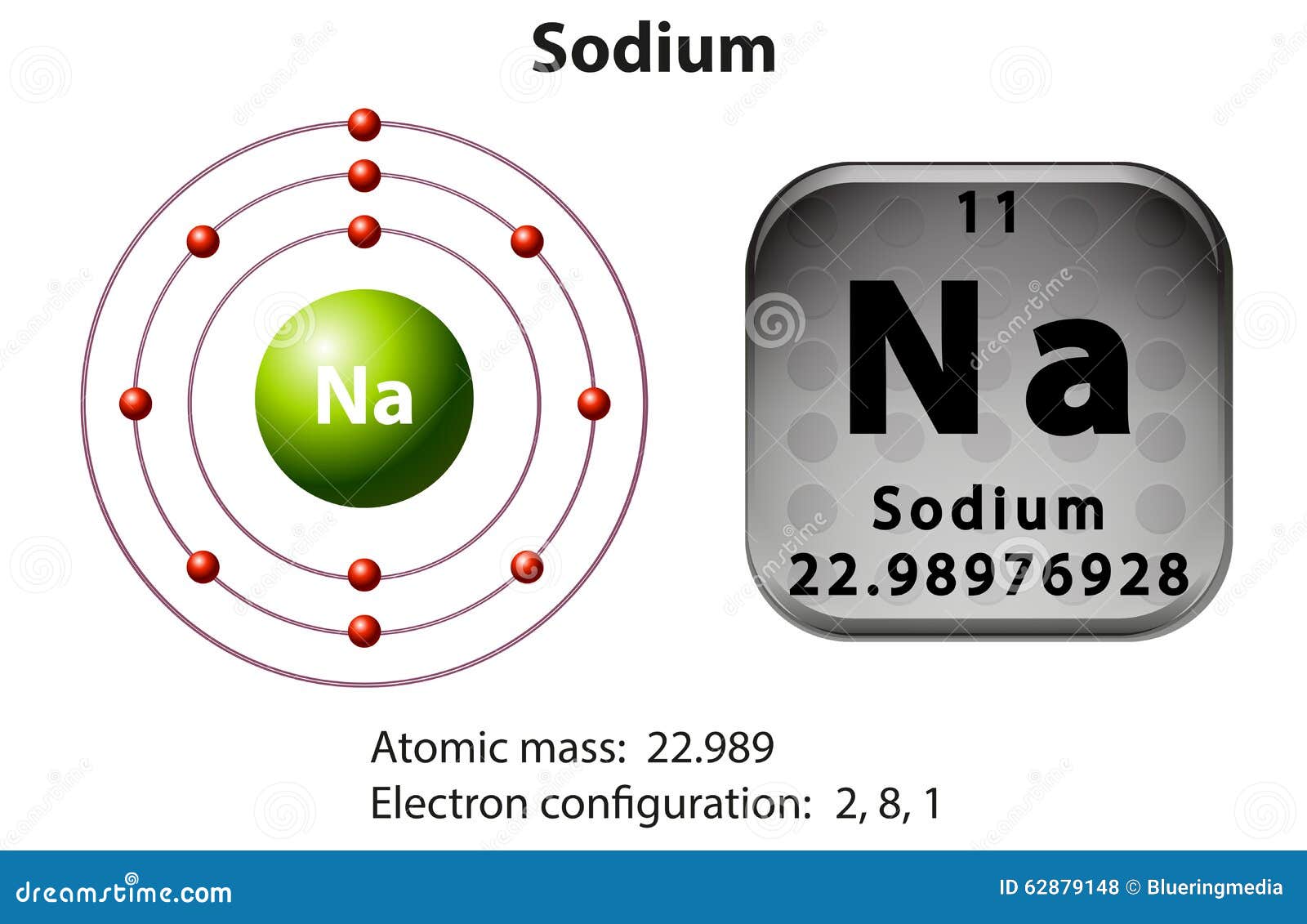
Symbol and Electron Diagram for Sodium Stock Vector Illustration of

FileElectron shell 011 sodium.png Wikimedia Commons

Diagram representation of the element sodium Vector Image

Atom sodium Royalty Free Vector Image VectorStock
To Do That We Need To Find The Number Of Electrons For The Na Atom (There.
Web As Shown, Helium Has A Complete Outer Electron Shell, With Two Electrons Filling Its First And Only Shell.
Similarly, Neon Has A Complete Outer 2N Shell Containing Eight Electrons.
The First Two Shells Should Accommodate 2 And 8 Electrons, Respectively, While The Third Shell Holds The Remaining Electron.
Related Post: